Journal of
eISSN: 2471-1381


Research Article Volume 1 Issue 2
1Department of Medicine, University of Indonesia, Indonesia
2Digestive Disease & GI OncologyCenter, Medistra Hospital, Indonesia
3Victorian Infectious Disease Reference Laboratory, Australia
4Department of Anatomical Pathology, University of Indonesia, Indonesia
Correspondence: Lesmana CA Rinaldi, Department of Medicine, University of Indonesia, Indonesia
Received: June 28, 2015 | Published: September 21, 2015
Citation: Lesmana CRA, Jackson K, Hammond R, et al. The association among serum quantitative hepatitis b surface antigen (qhbsag), intrahepatic HBV markers and liver fibrosis in chronic hepatitis b (chb) patients. J Liver Res Disord Ther. 2015;1(2):32-35. DOI: 10.15406/jlrdt.2015.01.00007
Background: Recently, quantitative HBsAg has been used as a predictor for successful antiviral therapy in chronic hepatitis B (CHB) patients. Intrahepatic HBV-DNA levels have also been tested to know their relevance with clinical manifestation. However, their associations with liver fibrosis are still debatable.
Objective: This study was aimed to evaluate the association between serum qHbsAg, serum HBV-DNA, intrahepatic HBV markers and hepatic fibrosis in CHB patients.
Method: A cross-sectional study was done on naïve CHB patients from September 2009 to June 2011. Quantitative serum HBsAg measurement was performed using the automated chemiluminescent microparticle immunoassay (Architect HBsAg QT assay, Abbott Laboratories, IL, USA). Serum qHBsAg was measured using the automated chemiluminescent microparticle immunoassay (Architect HBsAg QT assay, Abbott Laboratories, IL, USA). Intrahepatic cccDNA was measured quantitatively from biopsy specimen (QiAmp DNA Mini Kit, Qiagen, Germany). Values were log-transformed before being analyzed. Biopsy specimens should include at least 5 portal systems and 1.5cm length to be eligible for evaluation using the METAVIR score.
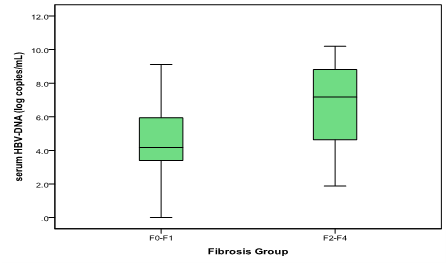
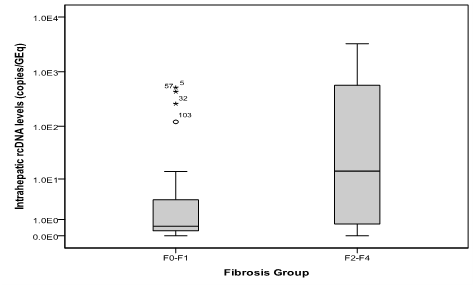
Results: 103 patients enrolled; 53(51.9%) of them were men. Mean age was 42+11.6 (range 19-70) years old. There were 60(58.3%) patients with HbeAg negative. Mean of log serum quantitative HBsAg was 2.54 for F0-F1 patients vs. 3.48 for F2-F4 patients (p<0.001, student t test). The mean log serum HBV-DNA was 4.65 for F0-F1 vs. 6.69 for F2-F4 patients (p<0.001; student t test). Intrahepatic rcDNA levels was higher in F2-F4 (median 14.37; range 0-3258.4 copies/GEq) than F0-F1 patients (median 0.50; range 0-514.4 copies/GEq); p<0.001 (Mann-Whitney U test). In contrast, virion productivity was not differed statistically between the two groups (p 0.096).
Conclusion: Quantitative serum HbsAg, HBV-DNA serum, and intrahepatic cccDNA are associated with fibrosis stage in chronic hepatitis B patients. HBV seromarkers might be used to predict disease severity.
Quantitative HBsAg has been widely used as a predictor for successful antiviral therapy in chronic hepatitis B (CHB) patients despite HBV DNA serum levels. Intrahepatic HBV-DNA levels have also been tested to know their relevance with clinical manifestation.1‒5 However, liver disease progression is also important to be routinely assessed especially if the patient has already advanced liver fibrosis. Liver biopsy is still the gold standard to assess liver inflammation and fibrosis despite other non-invasive tools.6Correlation between viral replication and liver disease progression is still questionable since Intrahepatic viral activity might not come along with the disease progression itself. The genetic predisposition and genotype distribution are the major factors that influence the liver disease progression.7,8 On the other side, ALT one of parameter for liver disease activity might not very useful since some studies have shown the prevalence of advanced liver fibrosis among CHB patients with normal ALT.9,10 This study was aimed to evaluate the association between serum qHBsAg, intrahepatic HBV-DNA levels and hepatic fibrosis in CHB patients.
Study design and subjects
This was a cross-sectional study conducted at two big referral hospitals from January 2010 to December 2011. All consecutive newly diagnosed CHB patients from Liver Outpatient’s clinic, Cipto Mangunkusumo and Medistra Hospitals, Jakarta who agreed to participate in the study after signing informed consent were enrolled. This study was approved by local ethics committee.
Chronic hepatitis B infection was diagnosed by the presence of HBsAg in the patients serum at least for 6 months based on chemiluminescent micro particle immunoassay (ARCHITECT HBsAg Reagent Kit, Abbott Diagnostics, Abbott Park, Illinois, USA). Inclusion criteria were newly diagnosed chronic hepatitis B patients who were willing to undergo liver biopsy for liver disease progression assessment. Patients were excluded if there were history of Hepatotoxicity drugs, co-infection with hepatitis C virus or human immunodeficiency virus, decompensate liver cirrhosis or hepatocellular carcinoma.
Quantitative HBV seromarkers measurements
Quantitative HBsAg was measured using chemiluminescent micro particle immunoassay (CMIA) method (ARCHITECT HBsAg Reagent Kit, Abbott Diagnostics, Abbott Park, Illinois, USA). The results were expressed as signal sample/ cutoff ratios (S/CO). The values were then calibrated (ARCHITECT® HBsAg Calibrators) and verified (ARCHITECT® HBsAg Controls) to obtain quantitative levels in IU/mL.
Serum HBV-DNA levels was measured with COBAS TaqMan HBV Test (Roche Diagnostics, Manheim, Germany). The detection range was ~30 U/mL to 1 x 108 U/mL (or 1.7 x 102 to 8.5 x 108 copies/mL). Samples were diluted and re-analyzed if the titer was above the upper limit of quantification.
Genotype measurement
Hepatitis B virus genotyping was measured using line probe assay (INNO-LiPA HBV, Innogenetics, Belgium).
Liver biopsy
Liver biopsy was done to all patients to obtain liver tissue specimen and histopathological assessment. It is performed using a 16-gauge Menghini needle (Hepafix®, B Braun Melsungen AG, Melsungen, Germany) under local anesthesia and ultrasound guided. Tissue specimens were first prepared for histopathology examination and the rest was stored in -80oC for genomic analysis. The examination was performed by experienced liver pathologist who blinded to clinical data. Fibrosis stage was assessed using the METAVIR scoring system.11,12
Intrahepatic total and cccDNA quantification
Intrahepatic HBV quantitative was measured at the Victorian Infectious Diseases Reference Laboratory, North Melbourne, Australia. The method has been previously established and reported.13 In brief, DNA extraction was performed using the tissue lysis protocol with the QIAamp DNA Mini Kit® (QIAGEN, Germany). Determination of HBV cccDNA levels was carried out by real-time PCR using the LightCycler® instrument (Roche Diagnostics, Mannheim, Germany). Reaction volumes of 20 ul consisted of 2 ul of extracted DNA, 5mM MgCl2, 0.5 uM of primer mix and 0.2 uM of hybridization probes. Selective HBV cccDNA primers consisted of two upstream primers, CCC1 5’-GCGGWCTCCCCGTCTGTGCC-3’ and CCC3 5’-GTCTGTGCCTTCTCATCTGC-3’ and the downstream primer, CCC2 5’-GTCCATGCCCCAAAGCCACC-3’. FRET hybridization probes were 5’-GTTCACGGTGGTCTCCATGCGACGT-FL-3’ and 5’LCR640-AGGTGAAGCGAAGTGCACACGGWCC-3’. Total intrahepatic DNA was measured using primers RC1 5’-CTCGTGGTGGACTTCTCTC-3’ and RC2 5’-CAGCAGGATGAAGAGGAA-3’ and FRET probes 5’- CACTCACCAACCTSYTGTCCTCCAA-FL-3’ and 5’LCR640-TGTCCTGGYTATCGCTGGATGTGTCT-3’. Quantification standards were derived by dilution of a linearized plasmid containing a greater than full length HBV genome. This had been previously titrated against the WHO international HBV reference standard to correlate quantification values. Variation in the amounts of liver tissue was normalized by quantifying ß-globin in each sample with the Roche DNA control kit (Roche Diagnostics). The relaxed circular HBV DNA was calculated as total HBV-DNA minus cccDNA.
Statistical Analysis
Characteristics of the study subjects were presented descriptively as proportion or median. Mean difference was tested using the student t test for normally distributed data. Median difference was tested on skewed data using the Mann-Whitney U test. A p value of less than 0.05 was considered significant. Analyses were done using the statistical software SPSS version 15 for Windows PC (SPSS Inc., Chicago, Illinois, USA).
There were 103 cases enrolled during the study period. Mean age was 42 + 11.6 years old, ranging from 19 to 70 years old. Sixty (58.3%) cases were HBeAg-negative (Table 1). Patients with significant fibrosis had higher serum quantitative HBsAg and HBV-DNA levels (Figure 1 & 2).

Intrahepatic HBV infection markers were assayed in all patients. Since there is no consensus how to present data, the absolute number was used in this study. We found that total Intrahepatic DNA, cccDNA and rcDNA were significantly higher in patients with F2-F4 fibrosis (Figure 3-5). In contrast, virion productivity was not differ statistically (Figure 6).

Our study has shown that Intrahepatic and serum viral markers level has been associated with the of liver disease progression. To our knowledge, this is the first study in Asia with large number of CHB patients who predominantly genotype B which showed the comprehensive association between liver histology, Intrahepatic HBV markers and HBV sero markers.
Based on natural history of CHB infection, young patients with HBV DNA levels and normal ALT (NALT) levels are usually well-known as immuno tolerance phase group; however, in this study most of the patients have NALT levels. We have shown in our previous publication that most of our young CHB patients even with NALT already had advanced liver fibrosis. To differentiate between immuno tolerance phase and immuno active or immuno clearance phase might not be easy since the ALT level can be fluctuated for many years and the immuno clearance phase itself can be more than 10years.9,14,15 Based on APASL HBV guideline, liver histology assessment is needed for CHB patients with NALT before treatment decision.16
This study result has shown that quantitative HBsAg and HBV DNA serum levels could predict the severity of liver fibrosis. There have been many studies about non-invasive tools that can be used for liver fibrosis assessment in CHB patients,17‒19 however, looking at the Intrahepatic viral activity which also been shown in this study, the use of non-invasive assessment might not be the right tools to start antiviral therapy since it has been reported that there was a grey area which is liver biopsy is still needed to confirm the disease activity or progression. These findings have given a new insight to assess our CHB patients more comprehensively by putting all together between Intra hepatic viral activity and disease activity. However, based on our study results, quantitative HBsAg and HBV-DNA serum levels might be used for starting antiviral treatment without any liver histology assessment. A recent study has shown that there was declining of Intrahepatic cccDNA along with liver histology improvement after oral antiviral therapy.20
Interestingly, virion productivity was not differed between mild fibrosis group and significant fibrosis group suggesting that HBV sero markers are very important markers for CHB patients monitoring either with or without antiviral therapy. This study also has limitations. First, this was a cross sectional study where liver biopsy was taken in one time only. However, it would be difficult to perform serial liver biopsy in clinical practice. Second, we did not differentiate in each CHB phase of infection, but based on our study subjects, it was not easy to differentiate the CHB phase of infection and it is more important to decide when the treatment should be started to prevent liver cancer development. Further study is needed to find out the accurate cut off from HBV sero markers for antiviral treatment decision.
Quantitative serum HBsAg and intrahepatic cccDNA are associated with fibrosis stage in chronic hepatitis B patients. Higher quantitative serum HBsAg and intrahepatic cccDNA levels could reflect more progressive liver disease. HBV sero markers might be used to start antiviral treatment in CHB patients.
None.
Author declares that there is no conflict of interest.

©2015 Lesmana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.