Journal of
eISSN: 2376-0060


Research Article Volume 9 Issue 4
Head of Radiotherapy Unit, Northern Cancer Center, Chil
Correspondence: Alejandro Santini Blasco, Head of Radiotherapy Unit, Northern Cancer Center, Manuel Antonio Matta 1868, 3er piso, 1240000 Antofagasta, Chile
Received: November 07, 2022 | Published: November 25, 2022
Citation: Blasco AS. Treatment of brain metastases in lung cancer. J Lung Pulm Respir Res. 2022;9(4):87-92. DOI: 10.15406/jlprr.2022.09.00286
Lung cancer is the most frequent oncological disorder and has been the main topic in most oncological congresses. There have been countless changes in diagnosis and treatment, improvements in the diagnostic methods, more effective and less invasive curative care and improvements in systemic treatments (new drugs and molecular targets). In the whole therapeutic spectrum, radiotherapy plays a significant role and at the brain metastasis level the changes are very relevant. Brain metastases are the most frequent neuro-oncologic complication in lung cancer patients. Managing this situation has become increasingly complex in recent years. The results have been improving, not only in terms of responses to treatment but also in terms of an increasing reduction in side effects. This paper reviews the latest advances and current management of patients with brain metastases from lung cancer.
Brain metastases (BM) represent the most frequent neurological complication in cancer patients and is, in turn, the most frequent neuro-oncological condition.1 The incidence is approximately 20-30% of patients with lung cancer throughout its history.2,3 An improvement in systemic treatments, which has increased the survival of these patients, has meant that the frequency continues to climb. During the last few years, the care of these patients has become more complex, new, more effective and less toxic treatment techniques have been developed, techniques that are more adapted to each patient, and the integration of radiotherapy with systemic treatments has become more complex, more frequent and more varied. All these changes have caused this topic to go from being evaluated in radiotherapy texts in just one or two pages in 1980, to complete books in 2020. In this paper the key role of radiotherapy in the care of these patients, its approaches, objectives and outcomes are reviewed.4,5
Epidemiology, clinics, diagnosis and prognosis
Approximately 30% to 40% of lung cancer patients will present with BM throughout their life, which may be diagnosed at the same time as their primary diagnosis or later (metachronous). About one third of them are solitary metastases, i.e., without evidence of an extracranial lesion or with a controlled primary. Lung cancer accounts for 40% of cases of brain metastases.1,6
Metastases usually develop at the junction of white and gray matter, in the so-called "watershed zone" of arterial circulation, when the caliber of blood vessels narrows and act as traps. BM can be presented with focal or generalized symptoms and approximately one third are asymptomatic at the time of diagnosis.7 The most frequent symptoms are headache, focal neurological alterations, seizures or intracranial hypertension. When a patient with a history of cancer presents neurological symptoms, it is always necessary to think, in the first instance, of brain metastases.8–10
A contrast-enhanced MRI is the preferred method of diagnosis, since it can detect lesions as small as 1.9 mm.7,8 A patient with acute neurological symptoms should probably undergo a CT scan with a contrast agent, given its easiness and speed, however, MRI is the definitive method of choice for the diagnosis and evaluation of the number and size of the neurological lesions.10 Most metastases are located in the cerebral hemispheres (80%) and although there is no pathognomonic pattern, they are usually iso or hypointense in T1 and hyperintense in T2 and are enhanced with the contrast agent. A thorough evaluation should be performed in patients presenting with the condition, guided by questioning and physical examination.
The patient's general condition and extracranial disease are decisive in establishing a prognosis and appropriate treatment. Recently, in high-risk cases (small cell lung cancer or adenocarcinoma), the term MRI screening has been used for the early diagnosis of BM.11 Gaspar et al. analyze the Radiation Oncology Therapy Group (RTOG) experience of more than 1200 patients and describe an RPA (Recursive Partitioning Analysis) prognostic factor analysis that classifies patients into three categories (Table 1).12
Risk assessment of patients with brain metastates (RPA) |
|
Type I |
Age > 65 years old |
KPS >70 |
|
Controlled primary tumor |
|
No extracranial metastases |
|
Type II |
All patients not in Type I or III |
Type III |
KPS<70 |
Table 1 RPA, “Rcursive partitioning anáyisis”, Risk classification in patients with brain metastases. Extracted from Gaspar et al.11
Following the work of Gaspar et al.,10 several authors tried to improve it, but it was not until 2008 when Sperduro et al. published a new scale, called GPA (Graded Prognostic Assessment), which has remained in use with some modifications up to the present day.13–14 This study analyzes RTOG data from 1960 patients with BM and takes into account four parameters: age, KPS, number of brain metastases, and presence or absence of extra cerebral metastases. Each parameter is classified with a score of 0, 0.5 or 1 and the GPS is calculated as the sum of all. The GPA classification is divided into 4 groups, GPA 0-1 associated with a median survival of 2.6 months, GPA 1.5-2.5 with a median survival of 3.8 months, GPA 3.0 with a median survival of 6.9 months and GPA 3.5 -4.0 with a median survival of 11 months. This scale is less subjective, easier to use and is currently the most commonly employed in clinical practice (Table 2). More recently it was confirmed that the prognosis of patients with BM also depended on the origin of the primary tumor, so site-specific staging systems (DS-GPA) were developed.15
|
|
|
|
Prognostic factor |
0 |
0,5 |
1,0 |
Age |
>60 |
50-60 |
<50 |
KPS |
<70 |
70-80 |
90-100 |
Extra cerebral metastases |
Present |
Absent |
|
Number of brain metastases |
>3 |
02-Mar |
1 |
Score GPA |
Average survival (months) |
0-1,0 |
2,6 |
1,5-2,5 |
3.8 |
3 |
6,9 |
3,5-4 |
11,0 |
Table 2 Modified Sperdutto et al.15
Sperdutto et al.,15 performed a retrospective, multi-institutional analysis of more than 4000 patients with BM treated between 1997 and 2007. This analysis allowed the development of site-specific prognostic classification systems that show certain differences, for example, for melanoma and renal cancer, KPS and the number of brain metastases are more relevant than age and the presence or absence of extra cerebral disease, while for breast cancer and digestive tumors, KPS is the most important factor.16,17
Treatment
The initial treatment of a patient with suspected or confirmed BM is with corticosteroids, since they effectively improve edema, inflammation and neurological deficits in more than 60% of cases in the first 24-48 hours.18 However, in the absence of symptoms, the use of corticosteroids may be debated.12 The use of corticosteroids for the management of peritumoral edema dates back to 1950 and remains the most important group of drugs.19 Its anti-edema action is explained by vasoconstriction, reduction of leukotriene formation and VEGF expression through the expression of glucocorticoid receptors. Undoubtedly, it has undesirable effects the list of which is beyond the scope of this publication, but the clinical improvement in symptomatic patients is clearly evident. Several authors do not recommend its use in asymptomatic patients and the least effective dose should always be used.20 Dexamethasone is the corticosteroid of choice, given its low mineralocorticoid activity and low risk of infectious and neuropsychiatric complications. Generally, the use of 4-8 mg per day results in a significant improvement and in some cases of significant edema with mass effect, higher doses can be administered.21
While the plasma average life is 2h, the biological average life is 36-54h, which allows for twice daily dosing. Adequate titration is necessary to achieve the lowest possible dose, which usually takes 10-15 days.21 The use of corticosteroids should be accompanied by gastric protection, usually with proton pump inhibitors. In patients who remain on this treatment for a prolonged period of time (more than 4 weeks), prophylaxis of occasional infections is also recommended, usually Cotrimoxazole (160-800mg) three times a week.
The use of anticonvulsant drugs in a prophylactic way is not recommended according to current evidence, however, the use of levetiracetam 500mg every 12 hours is a frequent practice in our environment.22 Levetiracetam is better tolerated than phenytoin, phenobarbital or valproic acid.
Whole brain irradiation (WBI) and protection of the hypocampus
WBI, once the standard treatment for patients with BM, continues to be the usual treatment for some patients with diffuse metastases (+ of 5) and regular general condition. Its effectiveness and improvement of symptoms is well known, ranging from 70 to 90%.23 The objective is to administer an adequate dose to the whole brain and to treat both macroscopic and microscopic disease. Table 3 describes some of the work demonstrating the effectiveness of WBI and confining slight differences between the different fractionations. At present, the most commonly used scheme is 30Gy in 10 fractions 5 times a week and for patients with regular general condition and uncontrolled extra cerebral disease. 20Gy/5Fractions is an acceptable scheme, for class 3 patients, with deterioration of the general condition.24–28
Score GPA |
Survival (months) Adenocarcinoma |
Survival (months) No Adenocarcinoma |
0-1,0 |
6,9 |
5,3 |
1,5-2,5 |
13.7 |
9,8 |
3 |
26,5 |
12.8 |
3,5-4 |
46,8 |
|
|
Work reviewing whole-brain irradiation (WBI) |
||
Author and quotation |
Detail of fractionation |
Number of patients |
Survival |
Borgelt et al (RTOG)24 |
30Gy/10 |
233p |
21 weeks |
30Gy/15 |
217p |
18 weeks |
|
40Gy/15 |
233p |
18 weeks |
|
40Gy/20 |
228p |
16 weeks |
|
20Gy/5 |
447p |
15 weeks |
|
Haie-Meder et al25 |
25Gy/10 |
110 |
4,2 months |
36Gy/6 (a one-week Split) |
106 |
5,3 months |
|
Prietsman et al26 |
30gy/10 |
263 |
84 days |
12Gy/2 |
270 |
77 days |
|
Murray et al (RTOG)27 |
30Gy/10 |
213 |
4,5 months |
54,4Gy/34 (32Gy in fractions of 1.8 two per day and boost of 22.4 in fractions of 1.6Gy two per day). |
216 |
4,5 months |
|
Graham et al28 |
40Gy/20 |
57 |
6,1months |
|
20Gy/4 |
56 |
6,6 months |
Table 3 Modified from Sperdutto et al.15
The most important objection to WBI is linked to the neurocognitive toxicity of patients receiving such treatment, which, in the middle term, alters the quality of life.29–35 WBI is one of the most frequent causes of neurocognitive impairment in cancer patients. Dementia has been reported in 11% of patients one year after treatment. This effect is more significant in those treated with fractions greater than 4Gy or in those receiving RT with concomitant CTX.36,37 Other authors confirm that patients with a significant reduction in the size of metastases (greater than 45%) have a lower risk of neurocognitive deterioration, so it cannot be ruled out that the deterioration may also be secondary to disease progression.38 Remodeling in the N-methyl-D-aspartate receptor (NMDAR) is one of the mechanisms that may explain neurocognitive impairment. Such receptors are activated by glutamate and this system is linked to learning and memory, however, overexpression leads to neuronal death.
After RT, hippocampal cells reorganize their receptors and a decrease in the density of NMDA receptors and an increase in the density of GABA receptors is evident.29 The understanding of this pathophysiology has led to the use of memantine (a drug used for the treatment of dementia in Alzheimer's patients), an NMDA receptor agonist. Some authors have demonstrated in animal models that the use of memantine during irradiation is enough to avoid some alterations in synaptic physiology.29 In this regard, the RTOG published the results of protocol 0614 comparing the use of memantine vs. placebo together with total encephalic RT. This study demonstrates that those patients receiving memantine together with RT presented better cognitive function over time, with less alteration of memory and intellectual processing speed.30
Another mechanism that may explain cognitive impairment after brain RT is the reduction in the number of neural progenitors, with a reduction of neurogenesis in the hippocampal area, more precisely in the dentate gyrus.31,32 Recall that neurogenesis in the hippocampus is responsible for short-term memory. From a clinical point of view, protection of the hippocampal dentate gyrus has been evaluated as a way to reduce the neurotoxicity of RT (Figure 1). Andreas et al. published the first work in this regard, employing more complex techniques such as IMRT, and the results were published in 2014. These results were encouraging and showed a clinical improvement and a reduction of neurotoxic effects in the medium term.32
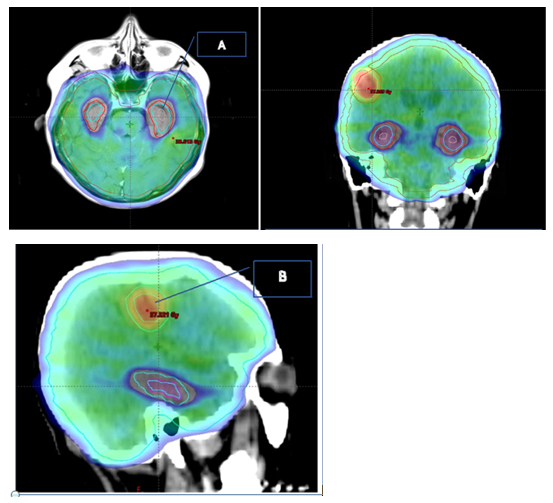
Figure 1 42-year-old patient with 3 metases of breast cancer. Treatment plan 30Gy/10 fractions to the brain, with protection of the Hippocampus(A) and concomitant boost up to 36GY in MRI-objectifiable metasis(B).
More recently, the use of RT with memantine was compared with hippocampal protection or not, and a better neurocognitive evolution was found in patients with protection without a difference in overall survival.33 Patients who were treated with radiotherapy and hippocampal protection reported less difficulty with memory, better ability to speak correctly, less interference with neurological symptoms. Yang et al. publish the result of a phase II study of RT with or without hippocampal protection and confirm an improvement in memory preservation at 6 months in the hippocampal sparing group.34
Based on these results, treatment with RT and hippocampal protection with memantine has been considered the standard for patients requiring whole brain irradiation.7,39–43 An interesting and novel technique is the use of simultaneous boost (SIB) during RT with hippocampal protection and memantine. Westover et al. treated 50 patients with WBI with hippocampal protection up to 20 Gy/10fractions with 40Gy/10F SIB in the overt lesions and report significantly better results than those obtained with WBI, comparable to modern SRS series.44-50,51
Role of surgery in the management of brain metastases
Surgery maintains its role in patients who do not have a histology or in those who have a voluminous mass, out of eloquent areas and require rapid decompression.9 Papers have been published evaluating the role of resection of a single metastasis and from these papers it can be concluded that resection should be reserved for large lesions, with large mass effect, or in patients who require a histological study and maintain a good general condition (KPS>70).39,40
Stereotactic radiotherapy or radiosurgery (SRS)
Stereotactic radiotherapy or radiosurgery is a highly sophisticated and precise technique based on the delivery of very high doses in one, or at most 5, radiation fractions, with submillimeter precision. Initially developed by the Swedish neurosurgeon Lras Leksell (1907-1986) with a machine called Gamma Knife, later techniques were developed to be able to perform it with modern linear accelerators.35,36 Initially also, used exclusively with complex stereotactic frames, it can now be done with simpler fixation instruments, what we call "frameless stereotactic mask-based approach” (Figure 2).
The main advantage of SRS is the possibility of protecting or avoiding irradiation of healthy brain parenchyma, and consequently reducing side effects.41 One of the problems that has been raised is the risk of progression of cerebral myco-metastases far from the area that is irradiated, which has been called distant brain recurrence.42 Some authors propose a close follow-up with MRI in patients treated with SRS, which would allow an eventual new treatment. SRS, initially relegated to very few specialized centers and for patients with a limited number of metastases, has become increasingly popular and today many centers have the technology for it.
It was initially employed as a boost after WBI and was later used exclusively. One of the first RTOG protocols, 95-08, included patients with 1 to 3 brain metastases and demonstrated a benefit in overall survival for patients with 1 metastasis but not for those with 2 or 3.42 In the same sense, Kondziokla et al. demonstrate the same for patients with 2 to 4 lesions.43,44
Chang et al. compare SRS treatment alone with SRS plus WBI and find that SRS and WBI leads to greater neurocognitive impairment.45 The same result is reached by Brown et al with the phase III study, N05754, which included patients with 1 to 3 metastases and compared SRS + WBI with SRS alone. Without a reduction in overall survival, patients who received SRS alone had less neurocognitive impairment after treatment.46
The recent Cochrane systematic review, which analyzed 54 papers, concluded that the addition of WBI could improve local control and reduce distant brain relapse in a selected group of patients, but with greater neurocognitive impairment and no change in overall survival. In patients with lung cancer and multiple brain metastases, the addition of WBI does not change the results, so omission of WBI is recommended.47 This same study confirms that the use of radiosensitizers, chemotherapy or treatment with target molecules is still experimental. For these authors, the role of neurological protectors, such as memantine or hippocampal protection, should also be confirmed.
As a result of these studies, most authors recommend the use of SRS alone for patients with a limited number of brain metastases, although in many centers this limited number of metastases is increasingly relative and the cut-off number for the number of lesions is not yet defined7. Other authors suggest a volume limit for the use of SRS. Several authors also describe acceptable results with SRS reirradiation for patients presenting with new brain metastases.48,49 To try and answer all these questions, the Canadian group started a randomized phase III trial for patients with 5 to 15 metastases comparing SRS vs WBI, with or without hippocampal protection, they estimate to recruit 206 patients and the results will be presented during 2023. Details of the protocol can be reviewed at the following link. (https://clinicaltrials.gov/ct2/show/NCT03550391)
A guideline jointly developed by ASCO (American Society of Clinical Oncology), SON (Society of Neuro-Oncology) and ASTRO (American Society of Radiation Oncology) for the management of these patients was recently published.50 In summary, this recommendation concludes the following:
ASTRO is also developing a guideline (to be published in 2022) that further elaborates and personalizes the treatment where treatment decision algorithms classify patients in a complex manner under a number of numerous parameters, among which the following stand out:
Technical aspects of srs
SRS can be performed with dedicated equipment such as the Gamma Knife or CyberKnife, or with equipment that can be used for SRS or radiation therapy such as linear accelerators. With all these techniques, appropriate fixation-immobilization accessories must be used, either with a stereotaxic frame or not (frameless) for treatment simulation. It is essential to have all relevant images, such as MRI, for image fusion and delimitation of treatment volumes, as well as Organs at Risk (OAR). Once defined, the fractionation scheme is decided according to the number and size of the lesions. The following table details the recommendations of the RTOG (Table 4).
Standard SRS fractionation scheme according to diameter, volume and location of lesions, Modified from Show et al.52 and Yamamamoto et al.53 |
|
Lesion smaller than 2 cm |
20-24Gy 1 F |
2 to 3 cm lesion |
18Gy 1 F |
Lesions larger than 3 cm |
15-16 Gy 1 F |
Lesions <4 cc |
22Gy |
Lesiones from 4 to 10 cc |
20Gy |
Brainstem lesions <1cc |
20Gy |
Lesions 1-4cc |
18Gy |
Lesions 4-10cc |
16Gy |
Table 4 Analysis of papers evaluating whole brain irradiation (WBI)
Once the plan is in place, treatment is performed. It is important to have portal images, ideally CBCT (cone beam) that must be evaluated in real time by the treating radiation oncologist and the medical physicist. The total treatment time on the machine is usually 30 to 90 minutes. The treatment is usually ambulatory, and the patient is monitored one week after treatment. Patients should usually have an imaging follow-up, ideally with MRI every three months for the first year and periodic neuropsychological evaluation.53
The therapeutic management of brain metastases in lung cancer patients, the most frequent complication from the neurological point of view, has significantly changed. A single, generalized treatment with discouraging results has given way to increasingly tailored treatment with encouraging results and involving a complex discussion adapted to each patient. New technology makes it possible to improve results and minimize side effects and maintain a better quality of life.
None.
The authors declare that they have no conflict interests.
None.

©2022 Blasco. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.