Journal of
eISSN: 2376-0060


Research Article Volume 8 Issue 2
1Division of Acute Care Surgery, Department of Surgery, Rutgers Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA
2Rutgers Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA
3Department of Biostatistics and Epidemiology, Rutgers School of Public Health, New Brunswick, New Jersey, USA
Correspondence: Rachel L. Choron, MD, Assistant Professor, Department of Surgery, Rutgers Robert Wood Johnson Medical School, 125 Patterson Street, Suite 6300, New Brunswick, NJ.
Received: May 05, 2021 | Published: May 20, 2021
Citation: Choron RL, Iacono SA, Cong A, et al. The correlation of respiratory system compliance and mortality in COVID-19 acute respiratory distress syndrome: do phenotypes really exist? J Lung Pulm Respir Res. 2021;8(2):67-74. DOI: 10.15406/jlprr.2021.08.00253
Background: Recent literature suggests respiratory system compliance (Crs) based phenotypes exist among COVID-19 ARDS patients. We sought to determine whether these phenotypes exist and whether Crs predicts mortality.
Methods: A retrospective observational cohort study of 111 COVID-19 ARDS patients admitted March 11-July 8, 2020. Crs was averaged for the first 72-hours of mechanical ventilation. Crs<30ml/cmH2O was defined as poor Crs(phenotype-H) whereas Crs≥30ml/cmH2O as preserved Crs(phenotype-L).
Results: 111 COVID-19 ARDS patients were included, 40 phenotype-H and 71 phenotype-L. Both the mean PaO2/FiO2 ratio for the first 72-hours of mechanical ventilation and the PaO2/FiO2 ratio hospital nadir were lower in phenotype-H than L(115[IQR87] vs 165[87], p=0.016), (63[32] vs 75[59], p=0.026). There were no difference in characteristics, diagnostic studies, or complications between groups. Twenty-seven (67.5%) phenotype-H patients died vs 37(52.1%) phenotype-L(p=0.115). Multivariable regression did not reveal a mortality difference between phenotypes; however, a 2-fold mortality increase was noted in Crs<20 vs >50ml/cmH2O when analyzing ordinal Crs groups. Moving up one group level (ex. Crs30-39.9ml/cmH2O to 40-49.9ml/cmH2O), was marginally associated with 14% lower risk of death(RR=0.86, 95%CI 0.72, 1.01, p=0.065). This attenuated (RR=0.94, 95%CI 0.80, 1.11) when adjusting for pH nadir and PaO2/FiO2 ratio nadir.
Conclusion: We identified a spectrum of Crs in COVID-19 ARDS similar to Crs distribution in non-COVID-19 ARDS. While we identified increasing mortality as Crs decreased, there was no specific threshold marking significantly different mortality based on phenotype. We therefore would not define COVID-19 ARDS patients by phenotypes-H or L and would not stray from traditional ARDS ventilator management strategies.
Keywords: COVID-19, corona virus, acute respiratory distress syndrome, respiratory system compliance, pulmonary compliance, mortality, intensive care unit, critical care, mechanical ventilation
The coronavirus (COVID-19) pandemic was first reported in Wuhan, China in December 2019 and rapidly spread worldwide.1,2 As of April 21st, 2021, there have been over 144 million cases globally, including 31 million cases and 569,000 deaths in the United States.3 Mortality in intensive care unit (ICU) patients with COVID-19 remains high despite advances in treatment strategies. Mortality ranges from 30-60% among COVID-19 ICU patients and is even higher in patients requiring mechanical ventilation.4-9
A large percentage of COVID-19 patients who develop respiratory failure and hypoxemia have been diagnosed with acute respiratory distress syndrome (ARDS) and treated accordingly; however, few data exist correlating respiratory pathophysiology to clinical features and ventilator mechanics in COVID-19. Patients presenting with non-COVID-19 ARDS typically have low pulmonary compliance and loss of aerated tissue available for ventilation.10,11 While most patients with COVID-19 ARDS present similarly to non-COVID-19 ARDS, as defined by the Berlin Criteria,12 it has been postulated that some COVID-19 patients do not fit the classic ARDS phenotype characterized by poor pulmonary compliance. Several studies have demonstrated that some hypoxemic COVID-19 patients with respiratory failure requiring mechanical ventilation have higher than expected pulmonary compliance when compared to non-COVID-19 ARDS patients.13-15
Based on these observations, Gattinoni et al. proposed two distinct phenotypes among patients with COVID-19 pneumonia who met the Berlin criteria for ARDS.16 The proposed “L phenotype” was defined as low elastance and high compliance; conversely the “H phenotype” was defined as high elastance and low compliance.16 It was further hypothesized that unlike H phenotype patients who benefit from classic ARDS lung protective ventilator strategies, L phenotype patients would paradoxically benefit from low PEEP and high tidal volume to compensate for increased dead space caused by hypoxic pulmonary vasoconstriction, which is contrary to traditional ARDS management.
Our primary objective was to determine whether respiratory system compliance (Crs) based phenotypes still exist among COVID-19 ICU patients during the first 72-hour period after the initiation of mechanical ventilation in our patient cohort. Our secondary objective was to determine whether Crs was related to mortality.
Patient population and setting
This was a retrospective observational study of the first 111 consecutive COVID-19 patients admitted to the ICU who required mechanical ventilation. Patients were admitted from March 11, 2020 to July 8, 2020. The final study follow-up date was November 23rd, 2020. The Institutional Review Board approved this study. Patients who met inclusion criteria were 18 years of age or older, admitted to the ICU, SARS-CoV-19 positive confirmed by polymerase chain reaction (PCR) testing of nasopharyngeal swab, mechanically ventilated for respiratory failure, diagnosed with ARDS as per the Berlin definition,12 and discharged from the ICU or died at the final date of follow-up. The exclusion criterion was COVID-19 ICU patients who did not require mechanical ventilation. This study was performed in a 297-bed community hospital in Central New Jersey that is affiliated with a large academic tertiary care center. During the height of the pandemic, the ICU was expanded to three times its typical capacity during mid-April.
Data collection and definitions
Patient information was collected from the electronic medical record (Allscripts-Sunrise Clinical Manager, Chicago, IL). The data collected included patient demographics, past medical history, vital signs, laboratory testing, therapies utilized for treatment of COVID-19, ICU length of stay, hospital length of stay, days to initiation of mechanical ventilation, days of mechanical ventilation, mortality, and disposition. Complications collected included acute respiratory distress syndrome (ARDS) defined by the Berlin criteria,12 acute kidney injury as defined by the Kidney Disease: Improving Global Outcomes (KDIGO) definition,17 culture proven infection, image proven venous thromboembolism, hemorrhagic events, and need for tracheostomy.
Ventilator data, arterial blood gases, and respiratory mechanics were collected during the first 72 hours following intubation for each patient. The Crs (ml/cm H2O) was defined as the tidal volume (VT) divided by the difference between plateau pressure and positive end expiratory pressure (PEEP). Average Crs was obtained for each patient over the first 72 hours they were mechanically ventilated. Crs<30 ml/cm H2O was defined as poor Crs or phenotype H whereas Crs≥30 ml/cm H2O was defined as preserved Crs or phenotype L.
All critically ill COVID-19 patients were cared for by a multidisciplinary team led by a board certified intensivist 24 hours a day seven days a week. Clinical practice patterns involved protective lung ventilation strategies as recommended by the ARDS network, however as this was a retrospective observational study without intervention, practice patterns were left to the discretion of the attending intensivist.
Statistical analysis
The distributions of baseline characteristics, vital signs, laboratory results, treatments, and outcomes were calculated for the entire study population, for phenotype H patients with Crs<30ml/cm H2O, and for phenotype L patients with Crs≥30 ml/cm H2O. Frequency and percentages are reported for categorical variables, and median and interquartile range (IQR) are reported for continuous variables, since nearly all were determined to be nonnormally distributed according to the Shapiro-Wilk statistic. Bivariate comparisons of categorical and continuous variables were tested using Pearson’s chi-square statistic (or Fisher’s exact test when warranted by small cell counts) or two-sided Wilcoxon Rank-Sum statistics, respectively.
Mortality along with other continuous variable outcomes (hospital days prior to mechanical ventilation, mechanical ventilator days, ICU length of stay, and hospital length of stay) were compared for phenotypes H and L. To determine whether a Crs threshold other than 30ml/cm H2O impacted mortality and outcomes, patients were divided into five ordinal groups (i.e., <20, 20 to <30, 30 to <40, 40 to <50, and >50 ml/cm H2O) based on Crs and Kruskal-Wallis test was used to assess bivariate association with continuous variable outcomes. Adjusted relative risks were computed using multivariable modified (robust variance estimator) Poisson regression models; first analysis was with Crs≥30 ml/cm H2O vs <30ml/cm H2O (Model 1), adjusted for pH nadir<7.2 and lowest PaO2/FiO2 Ratio (Model 2), the second analysis evaluated the 5-level ordinal Crs groups. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
At the final date of follow-up, 111 COVID-19 positive ICU patients met study inclusion criteria which included requiring mechanical ventilation and meeting the Berlin criteria for ARDS. The median age for the overall population was 64 years (IQR 17) and the majority were male (76, 68.5%). Most patients were Caucasian (50, 45.1%), followed by Hispanic (39, 35.1%), and Black (15, 13.5%) (Table 1A). Of the total population, 40 patients (36%) were classified as phenotype H (Crs<30 ml/cm H2O) and 71 (64%) were classified as phenotype L (Crs≥30 ml/cm H2O).
|
|
All COVID-19 |
Patients with Crs |
Patients with Crs |
p-value |
|
Age, median (IQR), years |
64 (17) |
66 (14.5) |
61 (16) |
0.092 |
|
Sex, male (n, %) |
76 (68.5) |
22 (55) |
54 (76.1) |
0.022 |
|
Race/Ethnicity |
|
|
|
|
|
Caucasian |
50 (45.1) |
20 (50) |
30 (42.3) |
0.645 |
|
Black |
15 (13.5) |
6 (15) |
9 (12.7) |
|
|
Hispanic |
39 (35.1) |
11 (27.5) |
28 (39.4) |
|
|
Asian |
7 (6.3) |
3 (7.5) |
4 (5.6) |
|
|
Body Mass Index, median (IQR), kg/m2 |
30.8 (10.3) |
29.4 (9.5) |
30.8 (10.1) |
0.338 |
|
Comorbities, (n, %) |
|
|
|
|
|
Diabetes |
49 (44.1) |
20 (50) |
29 (40.9) |
0.351 |
|
Hypertension |
68 (61.3) |
28 (70) |
40 (56.3) |
0.156 |
|
COPD/Asthma |
11 (9.9) |
4 (10) |
7 (9.9) |
1.000 |
|
Cardiovascular Disease (CAD, MI, HF) |
26 (23.4) |
11 (27.5) |
15 (21.1) |
0.449 |
|
Smoking History |
22 (19.8) |
7 (17.5) |
15 (21.1) |
0.645 |
|
Chronic Kidney Disease |
14 (12.6) |
3 (7.5) |
11 (15.5) |
0.223 |
Table 1a Characteristics of mechanically ventilated ARDS patients with COVID-19 stratified by Crs
The most common comorbidities among all COVID-19 ICU patients were hypertension (61.3%), diabetes (44.1%), and obesity with a median body mass index 30.8 kg/m2 (IQR 10.3). Baseline characteristics, listed in Table 1A, were similar among phenotypes H and L. Admission vital signs and laboratory values were also similar between the two groups (Table 1B). While not statistically significant, phenotype H had a lower heart rate compared to phenotype L (94.5 beats per minute [IQR25] vs 101 [29], p=0.059) and phenotype H trended toward a lower mean arterial pressure (89 mmHg [26] vs 95 [21], p=0.057).
|
Admission Vital Signs, median (IQR) |
|
|
|
|
|
Temperature, degrees Fahrenheit |
99.8 (3.5) |
99.6 (3.2) |
100 (4) |
0.638 |
|
Heart Rate, beats per minute |
99 (25) |
94.5 (25) |
101 (29) |
0.059 |
|
Systolic Blood Pressure, mmHg |
132 (32) |
129.5 (35.5) |
134 (31) |
0.174 |
|
Mean Arterial Pressure, mmHg |
93.5 (21) |
89 (26) |
95 (21) |
0.057 |
|
Initial O2 Saturation |
89 (13) |
88 (13) |
90 (12) |
0.306 |
|
Admission Laboratory Results |
|
|
|
|
|
White Blood Cell Count, x109/L |
8.2 (5.8) |
7.1 (6.1) |
8.4 (6.1) |
0.253 |
|
Absolute Lymphocyte Count, x109/L |
5.9 (6.9) |
5.9 (6.2) |
5.9 (7.8) |
0.849 |
|
Sodium, mmol/L |
135 (6) |
135.5 (6) |
135 (6) |
0.941 |
|
Creatinine, mg/dL |
1.0 (0.7) |
1.0 (0.9) |
1.0 (0.7) |
0.927 |
|
Hemoglobin, g/dL |
13.2 (2.8) |
13 (3) |
13.2 (2.8) |
0.386 |
|
Platelets, x 109/L |
213 (133) |
205 (95) |
225 (148) |
0.512 |
|
Prothrombin Time, sec |
10.9 (1.5) |
10.8 (0.9) |
11 (1.8) |
0.573 |
|
Admission Studies |
|
|
|
|
|
Bilateral Infiltrates on Chest X-ray, n (%) |
101 (91) |
37 (92.5) |
64 (90.1) |
1.000 |
|
Highest Value During Hospitalization |
|
|
|
|
|
Lactate Dehydrogenase, U/L, median (IQR) |
569 (329) |
515 (378) |
612 (326) |
0.069 |
|
Ferritin, ng/mL |
1161 (1372) |
950 (1346) |
1185 (1421) |
0.449 |
|
Triglycerides, mg/dL |
216 (227) |
187 (178) |
225 (221) |
0.257 |
|
D-Dimer, mg/L |
5.5 (13.8) |
4.2 (10.9) |
6.4 (18.8) |
0.225 |
|
Fibrinogen, mg/dL |
634 (269) |
624 (223) |
655 (232) |
0.319 |
|
Gas Exchange |
|
|
|
|
|
Mean PaO2/FiO2, mmHg for 1st 72 hours during IMV |
147 (94) |
115 (87) |
164 (87) |
0.016 |
|
Lowest PaO2/FiO2, mmHg during IMV |
73 (42.3) |
63 (32) |
75 (59) |
0.026 |
|
Mean pH for 1st 72 hours during IMV |
7.4 (0.1) |
7.4 (0.1) |
7.4 (0.1) |
0.522 |
|
Lowest pH during IMV |
7.2 (0.2) |
7.2 (0.2) |
7.2 (0.3) |
0.990 |
Table 1b Vital Signs, Laboratory Results, and Gas Exchange Data Stratified by Crs
Abbreviations: ARDS, acute respiratory distress syndrome; Crs, respiratory system compliance; IQR, interquartile range; CAD, coronary artery disease; MI, myocardial infarction; HF, heart failure; IMV, invasive mechanical ventilation
Both phenotype H and L patients were critically ill. The majority of patients had shock requiring vasopressors during ICU admission (88.9%). All patients had ARDS; 83 (74.8%) had severe ARDS based on Berlin criteria. The mean PaO2/FiO2 ratio for the first 72 hours of mechanical ventilation was significantly lower in phenotype H (115 [87] vs 165 [87], p=0.016). Likewise, the PaO2/FiO2 ratio nadir for the entire mechanical ventilation course was lower in phenotype H than L (63 [32] vs 75 [59], p=0.026).
Complications were similar between both phenotype H and L groups (Table 2). Fifty-one (46.4%) patients had concomitant bacterial pneumonia and 26 (23.4%) had bacteremia. Nearly half (52, 47.3%) had acute kidney injury and 32 (28.8%) required renal replacement therapy. There was no difference among infectious, cardiac, or other complications between the two groups. While not statistically significant, fewer phenotype H patients underwent tracheostomy procedures than phenotype L (2 [5%] vs 12 [16.9%], p=0.070).
|
|
All COVID-19 ARDS Patients |
Patients with Crs <30 ml/cm H2O Phenotype H (n=40) |
Patients with Crs ≥ 30 ml/cm H2O Phenotype L |
p-value |
|
|
n (%) |
n (%) |
n (%) |
|
|
ARDS |
|
|
|
|
|
Mild ARDS |
5 (4.5) |
1 (2.5) |
4 (5.6) |
0.416 |
|
Moderate ARDS |
23 (20.7) |
6 (15) |
17 (23.9) |
0.264 |
|
Severe ARDS |
83 (74.8) |
33 (82.5) |
50 (70.4) |
0.160 |
|
Vasopressor Requirement |
97 (88.9) |
36 (92.3) |
61 (87.1) |
0.532 |
|
Infectious Complications |
|
|
|
|
|
Bacterial Pneumonia |
51 (46.4) |
15 (37.5) |
36 (51.4) |
0.159 |
|
Urinary Tract Infection |
24 (21.6) |
10 (25) |
14 (19.7) |
0.516 |
|
Bacteremia |
26 (23.4) |
10 (25) |
16 (22.5) |
0.769 |
|
Influenza |
2 (1.8) |
0 |
2 (2.8) |
0.535 |
|
Clostridium Difficile |
2 (1.8) |
0 |
2 (2.8) |
0.535 |
|
Acute Kidney Injury |
52 (47.3) |
19 (48.7) |
33 (46.5) |
0.822 |
|
Kidney Replacement Therapy |
32 (28.8) |
10 (25) |
22 (31) |
0.504 |
|
Acute Hepatic Injury |
9 (8.1) |
5 (12.5) |
4 (5.6) |
0.279 |
|
Cardiac Complications |
|
|
|
|
|
Arrhythmia |
34 (30.6) |
8 (20) |
26 (36.6) |
0.068 |
|
Myocardial Infarction |
4 (3.6) |
1 (2.5) |
3 (4.2) |
1.000 |
|
Cardiomyopathy |
8 (7.2) |
2 (5) |
6 (8.5) |
0.709 |
|
Pneumothorax |
11 (9.9) |
3 (7.5) |
8 (11.3) |
0.743 |
|
Seizures |
2 (1.8) |
1 (2.5) |
1 (1.4) |
1.000 |
|
Deep Vein Thrombosis |
3 (2.7) |
0 |
3 (4.2) |
0.552 |
|
Pulmonary Embolism |
2 (1.8) |
0 |
2 (2.9) |
0.531 |
|
Tracheostomy |
14 (12.6) |
2 (5) |
12 (16.9) |
0.070 |
Table 2 Complications of mechanically ventilated ARDS patients with COVID-19 stratified by Crs
Abbreviations: ARDS, acute respiratory distress syndrome; Crs, respiratory system compliance; IQR, interquartile range
Outcomes and mortality as related to respiratory system compliance
The overall mortality was 57.7% for COVID-19 ARDS patients, with median mechanical ventilator days 10 [IQR11], ICU length of stay 12 days [12], and hospital length of stay 11 days [IQR 15] (Table 3A). There was no difference in ventilator days, ICU length of stay, hospital length of stay, or mortality when comparing phenotypes H and L. Twenty-seven (67.5%) phenotype H patients died as compared to 37 (52.1%) phenotype L patients (p=0.115). While there was no difference in mortality when stratifying respiratory system compliance based on phenotypes H and L, there was a trend toward increasing mortality with decreasing respiratory system compliance when dividing the patients into ordinal groups (Table 3B, Figure 1). There was 40% mortality among patients with Crs > 50 ml/cm H2O vs 55.1% mortality with Crs 30-39.9 ml/cm H2O and 80% mortality with Crs <20 ml/cm H2O.
|
|
All COVID-19 ARDS Patients |
Patients with Crs |
Patients with Crs |
p-value |
|
Hospital Days Prior to IMV, median (IQR) |
2.0 (3.0) |
1.0 (3.0) |
2.0 (4.0) |
0.823 |
|
Mechanical Ventilator Days |
10 (11) |
11.5 (9) |
9 (13) |
0.880 |
|
Intensive Care Unit Length of Stay, days |
12 (12) |
13.5 (10) |
12 (17) |
0.958 |
|
Hospital Length of Stay, days |
17 (15) |
16 (15) |
17 (16) |
0.305 |
Mortality, n (%) |
64 (57.7) |
27 (67.5) |
37 (52.1) |
0.115 |
|
Mortality among proned patients (n=31) |
22/31 (71) |
9/11 (81.8) |
13/20 (65) |
0.429 |
Table 3A Outcomes of mechanically ventilated ARDS patients with COVID-19 stratified by Crs
|
|
Crs <20 |
Crs 20-29.9 |
Crs 30-39.9 |
Crs 40-49.9 |
Crs >50 |
p-value |
|
Hospital Days Prior to IMV, median (IQR) |
2 (2) |
1 (3) |
1 (4) |
4 (5) |
2 (3) |
0.777 |
|
Mechanical Ventilator Days |
7 (3) |
12 (9) |
10 (12) |
8 (19) |
6 (16) |
0.681 |
|
Intensive Care Unit Length of Stay, days |
8 (4) |
14 (10) |
12 (14) |
8 (19) |
8 (21) |
0.707 |
|
Hospital Length of Stay, days |
11 (17) |
16 (14) |
17 (15) |
15 (15) |
18 (30) |
0.735 |
|
Mortality, n (%) |
4 (80) |
23 (65.7) |
27 (55.1) |
4 (57.1) |
6 (40) |
0.681 |
|
Mortality among proned patients |
1/1 (100) |
8/10 (80) |
11/15 (73.3) |
0 |
2/5 (40) |
|
Table 3B Outcomes of Mechanically Ventilated ARDS Patients with COVID-19 Based on Crs Group
Abbreviations: ARDS, acute respiratory distress syndrome; Crs, respiratory system compliance; IQR, interquartile range; IMV, invasive mechanical ventilation
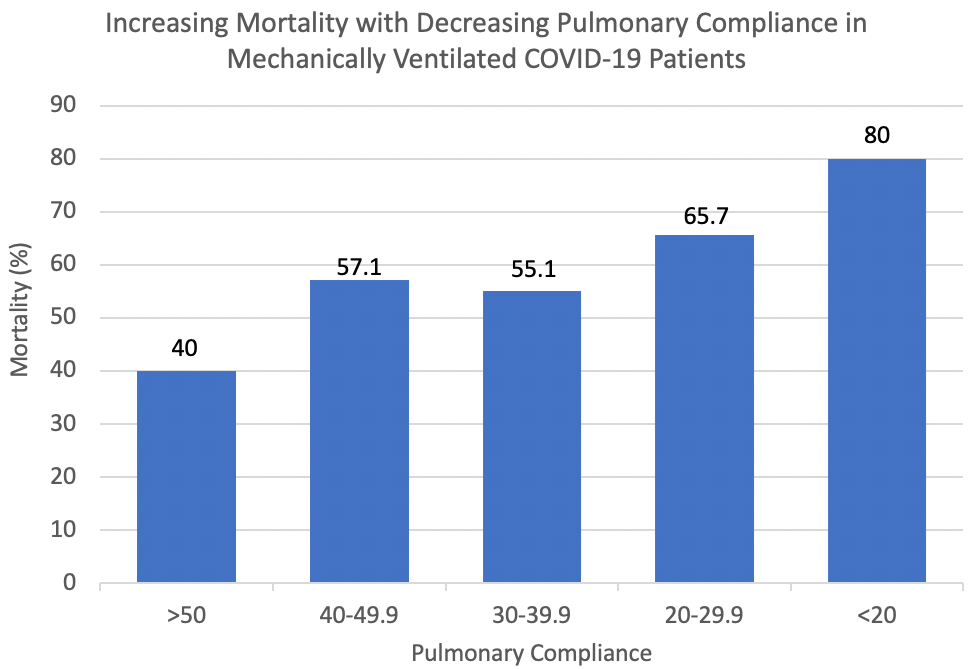
Figure 1 Increasing mortality demonstrated among mechanically ventilated COVID-19 ARDS patients with decreasing respiratory system compliance.
Multivariable analysis
In multivariable analysis models one and two, respiratory system compliance was defined as phenotype H and L. Using these models Crs was not associated with mortality and neither was PaO2/FiO2 ratio nadir; whereas abnormal pH was associated with a 2-fold increased risk for mortality (Table 4A). When evaluating Crs ordinally instead of as binary phenotype, a 2-fold increase in mortality was noted in Crs<20 vs > 50 ml/cm H2O (RR 2.00, 95% CI 0.94-4.27); however, this association did not hold upon adjusting for pH nadir and PaO2/FiO2 ratio nadir (Table 4B). Ordinally, each additional group level (ex. Crs 30-39.9 ml/cm H2O to 40-49.9 ml/cm H2O) was marginally associated with a 14% lower risk of death (RR=0.86, 95% CI 0.72, 1.01, p=0.065), this attenuates (RR=0.94, 95% CI 0.80, 1.11) when adjusting for pH nadir and PaO2/FiO2 ratio nadir.
|
|
Model 1 |
Model 2 |
||
|
|
RR |
95% CI |
RR |
95% CI |
|
Compliance, Crs >30 vs <30 ml/cmH2O |
0.77 |
0.57, 1.05 |
0.90 |
0.68, 1.19 |
|
pH Nadir <7.2 |
|
|
2.04 |
1.27, 3.28 |
|
Lowest PaO2/FiO2 Ratio |
|
|
0.99 |
0.99, 1.00 |
Table 4a Multivariable modified poisson regression analysis of mechanically ventilated ARDS patients with COVID-19
|
|
Model 3 |
Model 4 |
||
|
|
RR |
95% CI |
RR |
95% CI |
|
Compliance |
|
|
|
|
|
Crs <20 ml/cmH2O |
2.00 |
0.94, 4.27 |
1.16 |
0.50, 2.17 |
|
Crs 20-29.9 ml/cmH2O |
1.64 |
0.85, 3.19 |
1.31 |
0.67, 2.54 |
|
Crs 30-39.9 ml/cmH2O |
1.38 |
0.71, 2.69 |
1.19 |
0.62, 2.29 |
|
Crs 40-49.9 ml/cmH2O |
1.43 |
0.59, 3.49 |
1.22 |
0.51, 2.89 |
|
Crs >50 ml/cmH2O |
reference |
|
|
|
|
pH Nadir <7.2 |
|
|
2.05 |
1.27, 3.33 |
|
Lowest PaO2/FiO2 Ratio |
|
|
0.99 |
0.99, 1.00 |
Table 4b Multivariable modified poisson regression analysis of mechanically ventilated ARDS patients with COVID-19 based on ordinal group
*Ordinal trend: Model 3 RR 0.86 (95% CI 0.72-1.01, p=0.065); Model 4 RR 0.94 (95% CI 0.80-1.11, p=0.458)
Abbreviations: ARDS, acute respiratory distress syndrome; Crs, respiratory system compliance; RR, risk ratio; CI, confidence interval
While some argue that the mechanical properties of COVID-19 affected lungs are unique, recent studies have shown that patients with COVID-19 ARDS versus non-COVID-19 ARDS are similar.18-21 Prior to COVID-19, Crs had been correlated with ARDS as it affected the amount of aerated lung volume; however, the prognostic value of Crs on mortality remains unclear.22 Ultimately Crs was not included in the Berlin definition of ARDS secondary to the lack of evidence for its predictive validity.12 As the pandemic emerged, it was proposed that two phenotypes existed differentiating COVID-19 ARDS based on high vs low Crs. It was suggested this was atypical from non-COVID-19 ARDS. In reality, prior literature had not examined phenotypic variations of Crs in ARDS prior to the pandemic. This led Panwar et al. to perform a secondary analysis of the LUNG SAFE study to determine if Crs-based phenotypes exist in non-COVID-19 ARDS and whether Crs impacts outcomes.23 They found a wide range of Crs with one in eight patients having preserved Crs (Crs >50 ml/cmH2O). Among those phenotype L patients, a significant portion had moderate to severe hypoxemia. Lower Crs on the first day of ARDS, was independently associated with higher mortality, however there was no clear transition point suggesting arbitrary thresholds for phenotype definitions.23
Panwar’s findings among non-COVID-19 ARDS patients are comparable to our findings in the COVID-19 ARDS population. We also found a wide range of Crs with about one in eight patients having preserved Crs >50 ml/cmH2O. While we did see an increasing risk of mortality with decreasing Crs, we did not find a statistically significant difference in mortality when comparing phenotype H vs L as defined by Crs <30 vs >30 ml/cmH2O. Likewise, we did not find any clear transition threshold to define Crs phenotypes based on a mortality relationship.
Moving beyond diagnostic phenotyping of COVID-19 ARDS patients, it has also been proposed by proponents of the Crs phenotype concept that phenotype L patients should be managed with alternative ventilator strategies than the traditional approach to non-COVID-19 ARDS. These strategies would include low PEEP and high tidal volume to compensate for increased dead space caused by hypoxic pulmonary vasoconstriction.15,24 Following this postulation, several studies have confirmed heterogeneity of lung morphology among COVID-19 ARDS patients and concluded patient specific care along with an evidence-based approach to traditional ARDS management should remain the mainstay of COVID-19 ventilator management.25,26
Based on our findings, we propose the observed differences in compliance are part of a continuum of illness that is patient specific and not exclusive to or definable by specific phenotypes. We would continue to advocate for traditional lung-protective ventilation strategies in the COVID-19 ARDS population modified if needed by individual bedside assessment.21,27,28
While this is one of the largest case series to date analyzing Crs among mechanically ventilated COVID-19 ARDS patients, this is still a small single center series that was retrospective in nature. While our clinical practice pattern was adherence to lung protective ventilator strategies as recommended by the ARDS network, management was per the intensivist’s discretion and therefore may have had some heterogeneity. The collection of ventilation variables was limited to the first three days of mechanical ventilation following the onset of respiratory failure; we therefore cannot speak to the effect of Crs on mortality beyond day three of mechanical ventilation.
A wide spectrum of Crs was observed among COVID-19 ARDS patients requiring invasive mechanical ventilation. We found only 13.5% of these patients had preserved Crs > 50mL/cmH2O, which is consistent with the percentage of non-COVID-19 ARDS patients that have preserved Crs. While we identified a trend towards increasing mortality as Crs decreased, there was not an identifiable threshold marking a significant difference in mortality based on phenotypic definitions. We therefore would not define COVID-19 ARDS patients by phenotypes H or L and would not stray from traditional ARDS ventilator management strategies.
Funding: The authors did not receive support from any organization for the submitted work.
There are no conflicts of interest to declare.
There is transparency of data to support our manuscript.
There is custom code availability that supports our manuscript.
All authors made substantial contributions to conception, design, data collection and editing of the manuscript.

©2021 Choron, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.