Journal of
eISSN: 2376-0060


Research Article Volume 9 Issue 3
1National Facility for Biopharmaceuticals, Guru Nanak Khalsa College of Arts, Science, India
2School of Biotechnology and Bioinformatics, D Y Patil Deemed to be University, India
3Department of Biological Sciences, SVKM’s NMIMS Sunadan Divatia School of Science, India
Correspondence: Vikas Jha, National Facility for Biopharmaceuticals, Guru Nanak Khalsa College of Arts, Science & Commerce, Mumbai-400019, Maharashtra, India
Received: October 21, 2022 | Published: November 1, 2022
Citation: Jha V, Rane N, Kanade T, et al. Rv2746c and Rv2881c, a potential drug target of Mycobacterium tuberculosis revealed by in-silico investigation of proteins involved in lipid biosynthesis. J Lung Pulm Respir Res. 2022;9(3):66-73. DOI: 10.15406/jlprr.2022.09.00283
Tuberculosis is a serious disease that requires a greater understanding of its pathophysiology to develop effective treatment strategies. To gain a better understanding of mycobacterial physiology, researchers are focusing on the key components associated with cell wall synthesis. Although mycolic and fatty acids are the primary lipid components of the mycobacterial cell envelope, understanding the proteins involved in the lipid biosynthesis pathway may open up new avenues for fundamental research. This research included a thorough computational examination of proteins from the fatty acid biosynthesis pathways. Rv2881c and Rv2764c are essential genes for lipid synthesis. It is a potential drug target because knocking out these genes has an impact on Mtb growth. The study's findings provide researchers with specific cues and concrete information that can be applied in a variety of biotechnological applications.
Keywords: mycobacterium tuberculosis, lipid biosynthesis pathway, molecular docking, physio-chemical analysis, ligand interaction, drug target
Mtb, Mycobacterium tuberculosis; LAM, lipoarabinomannan; PDIM, phthiocerol dimycocerosate; DAT, diacyltrehaloses [GRAVY], grand average of hydropathicity; Mw, molecular weight; pI, isoelectric point; ESS, extended secondary structures; kcal/mol, kilocalorie per mole; ER, endoplasmic reticulum; cAMP, cyclic adenosine monophosphate; cGMP, guanosine 3',5'-cyclic monophosphate
Tuberculosis is a centuries-old disease that has evolved alongside humans in recent years.1 Despite numerous studies and new treatment modalities over the years, tuberculosis remains a threat to global public health. It is one of the top three infectious causes of death worldwide, according to the World Health Organization. Every year, approximately 10 million people contract tuberculosis (TB), with nearly 1.5 million dying as a result. However, the risk of developing active TB after infection is determined by several factors, one of which is the person's (host) immune response to it. Tuberculosis (TB) has also been observed to thrive wherever there is poverty, overcrowding, and chronic debilitating illness. Although tuberculosis remains one of the leading causes of death in resource-poor countries worldwide, the incidence of tuberculosis has increased in both developing and developed countries due to the emergence of drug-resistant strains.2
Mycobacterium tuberculosis (Mtb) pathophysiology suggests that various metabolic pathways are important in host-pathogen interaction, pathogenicity, and virulence.3 These pathways contain a slew of proteins that are essential for Mtb survival in the host. Mtb has a remarkable ability to persist in the host for long periods with a modified metabolic status and phenotypic drug tolerance without causing symptoms of active tuberculosis.4 Mtb cell wall, which is made up of mycolic acid, glycolipids such as diacyltrehaloses and polyacyltrehaloses, lipomannan, lipoarabinomannan (LAM), mannose-capped-LAM, sulfolipids, and trehalose-6,6'-dimycolate, gives it an advantage in the host.5 The glycobiology of Mtb infection of frontline alveolar macrophages is still unknown. Mycolic acid, Trehalose-6,6'-dimycolate, sulfolipid, phthiocerol dimycocerosate (PDIM), Diacyltrehaloses (DAT), and cholesterol are lipids that contribute to immune response suppression. Glycolipids such as LM, LAM, and Man-LAM modulate the host immune system and aid pathogen survival. Drug resistance occurs as a result of gene mutations in Mtb, most commonly as a result of an anti-TB drug.
Mtb changes its fatty acid metabolism to survive in the host, as evidenced by altered cell wall composition. LAM has been identified as an major virulence factor in tuberculosis because it is a major component of the cell wall and inhibits the activity of phosphatidyl-inositol-3-kinase, limiting phagosome maturation. According to legend, the M. tuberculosis H37Rv mutant strain that lacked phthiocerol dimycocerosates (PDIM) could not replicate and resulted in fewer tubercule formations. Mtb lipid homeostasis has a significant impact on host lipid metabolism. Lipids in the cell envelope contribute to virulence, survival, and persistence.6 The lipid metabolism of Mycobacterium tuberculosis is quite complicated. It is made up of catabolic and anabolic processes. Mtb produces a vast variety of lipids, ranging from simple fatty acids to extraordinarily complex long-chain substances like mycolic acid. About 250 enzymes are specifically specialized for fatty acid metabolism.7 Fatty acids are stored in the form of Triacylglycerol to counteract adverse situations such as nutrient deficiency.8 Triacylglycerol is related to the host's ability to support Mtb over the long run.9 Late diagnosis, improper or inadequate administration of effective medications, decreased availability of less toxic, less expensive, and effective medications, prolonged treatment duration, noncompliance with medication regimen, and development of drug resistance are the causes of the failure of TB therapy.
Several bioinformatics tools were used in this study to generate biochemical, structural, and functional information about all of the proteins involved in lipid biosynthesis. The goal of this study is to perform Insilco characterization on all of the proteins involved in this pathway and to elucidate their structural and functional details.
Retrieval of nucleotide and protein sequence
The protein's amino acid sequence was obtained using a Tuberculist. To gather the necessary sequences, several criteria, including gene name, location, etc., were applied.10 Information about each protein's function was gathered using the Mycobrowser server. A comprehensive proteome and genomic data repository for pathogenic Mycobacterium is called Mycobrowser, commonly known as the Mycobacterial browser. These organisms can be studied genomically and proteomically applying manually curated annotations to the given tools.11
Physiochemical characterization
The main structure of 25 proteins from lipid synthesis pathway was determined using ProtParam from ExPASy.12 It offers details on the physical and biochemical characteristics, such as the molecular weight (Mw), amino acid composition, extinction coefficients, isoelectric point, aliphatic index, instability index, number of negative and positive residues, half-life, and Grand average hydropathicity (GRAVY).13
Functional analysis
The CYS REC tool was used to predict the SS-bonding of cysteine residues and the placement of disulphide bridges in the protein sequence. The tool returns the position of the cysteines, their overall quantity, and, if any pairs of cysteines are present, their pattern within the protein sequence.14 The glycosylation sites were predicted using the NetNglyc service. The NetNglyc server predicts N-Glycosylation sites in human proteins by using artificial neural networks that consider the context of Asn-Xaa-Ser/Thr sequons. To describe the proteins and point out crucial areas, Inter-pro identifies protein families, domains, and functional sites.15 The amino acid sequence motifs were predicted using Psite.16
Human and gut flora non-homology analysis
Proteins with a high degree of homology to the host (the human) may exhibit undesired cross-reactivity and may obstruct the binding of the active sites. So, a non-homology BLAST search against the Human Proteome was carried out. Additionally, using a BLASTp search, the MTB lipid synthesis pathway proteins were checked against the proteins of the bacteria in the gut flora.17
Homology modelling
Tertiary protein structures give researchers crucial insight into the molecular processes that underlie protein activities, allowing them to design practical experiments like site-directed mutagenesis and examinations of mutation sites that are associated with the disease. The proteins were modelled in three dimensions using the SWISS-MODEL and PHYRE2 servers. A completely automated server called SWISS-MODEL provides comparative automated modelling of 3D protein structures. Similar to this, a 3D modelling service called PHYRE2 uses an Ab initio folding simulation called Poing2 to simulate parts of your proteins that don't match any known structures.
Structure validation
The 3D-modelled structures were validated using the SAVESv6.0 server. It offers several tools, including VERIFY3D, PROCHECK.18 The models in this study were authenticated using the PROCHECK tool. PROCHECK is a program that examines both the overall geometry of the protein as well as the geometry of each residue to validate the stereochemistry of a protein structure. Therefore, the ProSA-web application is used to calculate the predicted structure's overall model quality (Z-score), with the quality scores shown about all known protein structures. The QMEAN score was calculated to evaluate the structure's quality.19
Ligand retrieval
As ligands, Genistein, Coumestrol, Daidzein, Nyasol, and (Z)-hinokiresinol were chosen from the PubChem public chemical structure repository.20 These compounds' SDF files were downloaded and evaluated.
Protein and ligand preparation
Rv2881c and Rv2764c proteins were chosen from a list of 25 lipid synthesis pathway proteins. The 3D structures of these proteins were prepared for molecular docking analysis using the UCSF Chimera 1.15 tool, which added Kollman charges and polar hydrogen atoms to the protein molecule, eliminated the water molecules, and saved the charged protein molecule in PDB format.21
Ligand
As ligands, Genistein, Coumestrol, Daidzein, Nyasol, and (Z)-hinokiresinol were chosen, and their structures were obtained in SDF format from the PubChem databank. The PyRx Virtual Screening Tool was used to create structural variations to optimize and minimise the energy of the chosen ligands.22
Molecular docking
Molecular docking is an important method for predicting the prevailing binding interactions of a chosen ligand with a protein with a known three-dimensional structure. The six chosen ligand structures were docked with Mycobacterium tuberculosis Rv2881c and Rv2764c proteins using Auto Dock Vina.23 The Auto Dock Vina software performs bound confirmation prediction based on free binding energies computed using the empirical force field. The docking analysis was carried out using the Auto Dock Vina docking via PyRx Virtual Screening Tool.24 This analysis aided in the identification of candidate ligands with high protein binding affinities as potential inhibitors.
Physiochemical characterization
ExPAsy ProtParam Server was used to analyse the physiochemical parameters of 25 Mtb (protein involved in the process of lipid biosynthesis) Acyltransferase, Mycolytransferase, and Phospholipid biosynthesis proteins (Table 1). This service has analysis tools such as Compute pI/Mw, which is used to forecast the protein's isoelectric point (pI) and molecular weight (Mw); ProtParam, which is used to estimate numerous physicochemical characteristics; and PeptideMass, which is used to theoretically cleave proteins and calculate the masses of their peptides, as well as any known biological or artifactual posttranslational changes; PeptideCutter, which is used to anticipate cleavage sites of proteases or compounds present in protein sequences; ProtScale is a tool for representing amino acid scales, including hydrophobicity graphs. Protein molecular weight is directly related to the assessment of biomolecule functionality, such as gene and metabolic regulation.25 Rv2482c has the maximum molecular weight of 88282.96, while Rv2261c has the lowest molecular weight of 14923.09, according to the analysis. The isoelectric point (pI) of an amino acid is where the net charge is zero since it produces an equal quantity of positive and negative ions.26 The pI value of acidic amino acids is low, whereas the pI value of basic amino acids is high.27 Rv2746c was shown to be extremely basic with a value of 10.56, whereas Rv0914c was discovered to be acidic with a value of 4.99. In addition to the pI, the instability index (II) estimates the protein's stability in vitro and in vivo. If a protein's instability index is less than 40, it is stable; if it is greater than 40, it is unstable.28 Rv2482c, a protein involved in phosphatidylinositol synthesis, is the most unstable protein, with a score of 53.08; on the other side, Rv2612c, a protein involved in phosphatidylinositol synthesis, is the most stable protein, with a score of 24.5. Six (24%) of the proteins chosen were unstable, while 19 (76%) were stable. The aliphatic index is used to indicate the relative volume of a protein inhabited by aliphatic side chains (valine, leucine, isoleucine, and alanine). It is a measure of a protein's thermostability. A high aliphatic index protein is more heat stable.29 More over 40% of the proteins were thermally stable, with Rv2746c and Rv1822 being among the most stable and Rv3803c being the most unstable. The GRAVY value is used to calculate the average of Hydropathicity. A positive GRAVY value implies hydrophilicity and a non-polar protein, whereas a negative GRAVY value shows hydrophilicity and a polar protein with stronger water interaction.26 Rv2746c, with a value of 0.83, was clearly the most hydrophobic. Rv3720 has the lowest hydrophilicity (-0.362) and hence will interact with water the best.
Protein name |
Mol wt |
No of Amino acids |
pI |
Negative residues |
Positive residues |
Extinction coefficients |
Half life |
Aliphatic index |
Instability index |
GRAVY |
|||
Rv2289 |
28576.44 |
260 |
6.14 |
31 |
29 |
28420 |
28670 |
100 |
>20 |
>10 |
94.56 |
45.59 |
-0.152 |
Rv2881c |
32002.89 |
306 |
8.76 |
17 |
20 |
55710 |
55460 |
100 |
>20 |
>10 |
116.57 |
28.17 |
0.75 |
Rv3804c |
35686.2 |
338 |
6.08 |
25 |
23 |
73005 |
72880 |
30 |
>20 |
>10 |
73.08 |
40.28 |
-0.161 |
Rv1886c |
31890.91 |
300 |
5.69 |
21 |
19 |
68995 |
68870 |
30 |
>20 |
>10 |
73.3 |
46.47 |
-0.148 |
Rv0129c |
36771.27 |
340 |
5.92 |
23 |
21 |
85830 |
85830 |
30 |
>20 |
>10 |
68.97 |
49.34 |
-0.253 |
Rv3803c |
31088.89 |
299 |
6.13 |
18 |
15 |
83420 |
30 |
>20 |
>10 |
67.46 |
26.21 |
-0.049 |
|
Rv0564c |
36153.43 |
341 |
6.72 |
34 |
33 |
24075 |
23950 |
30 |
>20 |
>10 |
93.78 |
33.88 |
0.072 |
Rv2982c |
33992 |
334 |
5.49 |
30 |
25 |
24450 |
23950 |
30 |
>20 |
>10 |
102.37 |
43.66 |
0.296 |
Rv2612c |
23251.54 |
217 |
9.49 |
15 |
21 |
34740 |
34490 |
30 |
>20 |
>10 |
108.34 |
24.5 |
0.634 |
Rv1822 |
22911.34 |
209 |
9.85 |
8 |
15 |
58565 |
58440 |
30 |
>20 |
>10 |
120.81 |
30.07 |
0.72 |
Rv2746c |
22136.31 |
209 |
10.56 |
10 |
18 |
27960 |
27960 |
30 |
>20 |
>10 |
129.76 |
32.89 |
0.83 |
Rv1551 |
69191.98 |
621 |
6.1 |
84 |
78 |
77350 |
77350 |
100 |
>20 |
>10 |
101.58 |
40.21 |
-0.103 |
Rv2482c |
88282.96 |
789 |
8.53 |
97 |
100 |
96385 |
96260 |
100 |
>20 |
>10 |
90.58 |
53.08 |
-0.225 |
Rv0437c |
24227.95 |
231 |
9.48 |
20 |
25 |
10220 |
9970 |
100 |
>20 |
>10 |
100.61 |
41.65 |
0.148 |
Rv0436c |
31218.2 |
286 |
10.28 |
14 |
31 |
61670 |
61420 |
30 |
>20 |
>10 |
111.29 |
50.89 |
0.454 |
Rv0045c |
32099.06 |
298 |
5.24 |
39 |
25 |
35980 |
100 |
>20 |
>10 |
95.27 |
37.02 |
-0.106 |
|
Rv0914c |
43874.9 |
412 |
4.99 |
56 |
37 |
52160 |
52160 |
30 |
>20 |
>10 |
84.17 |
31.01 |
-0.2 |
Rv1543 |
36820.88 |
341 |
9.07 |
33 |
37 |
17545 |
17420 |
30 |
>20 |
>10 |
92.14 |
31.71 |
-0.084 |
Rv1627c |
42386 |
402 |
5.79 |
41 |
35 |
55265 |
54890 |
30 |
>20 |
>10 |
88.43 |
32.4 |
0.005 |
Rv1814 |
34882.39 |
300 |
9.69 |
21 |
31 |
110475 |
110350 |
30 |
>20 |
>10 |
95.3 |
32.1 |
-0.002 |
Rv1867 |
53560.49 |
494 |
5.58 |
60 |
50 |
79995 |
79870 |
30 |
>20 |
>10 |
85.77 |
34.58 |
-0.253 |
Rv2261c |
14923.09 |
140 |
8.82 |
9 |
11 |
21095 |
20970 |
5.5 |
3 |
2 |
98.36 |
39.43 |
0.355 |
Rv2262c |
37258.46 |
360 |
9.65 |
17 |
26 |
101325 |
100950 |
30 |
>20 |
>10 |
109.56 |
35.98 |
0.583 |
Rv3523 |
41421.29 |
394 |
6.2 |
40 |
37 |
60055 |
59930 |
30 |
>20 |
>10 |
83.1 |
36.58 |
0.001 |
Rv3720 |
46852.84 |
420 |
6.22 |
52 |
52 |
72100 |
71580 |
30 |
>20 |
>10 |
82.48 |
39.62 |
-0.362 |
Table 1 Physicochemical properties of proteins involved in lipid biosynthesis as predicted by Expasy’s protparam program
Functional analysis
The results of functional analysis using the CYS REC tool indicate that practically every protein possessed cysteine residues, with the exception of three proteins (Table 2): Rv3803c, Rv0045c, and Rv1627c. Cysteine is used in primary structure analysis because it is easily oxidised, resulting in a dimer with a disulphide bond between two cysteines. This property is required for the tertiary and quaternary structure to be stable.30 Table 2 shows that a total of four residues, cys13, cys315 from protein Rv3804c and cys127, cys132 from Rv1886c, are SS bound. N-glycosylation is one of the most difficult and crucial chemical reactions. The NetNGlyc programme predicted that 8 proteins do not contain any N-glycosylation sites (Table 3). Rv0129c has the most such sites of any protein in the sample. The Psite server, like the NetNGlyc programme, predicts the motifs contained in the selected amino acid sequences. N-myristoylation sites were found in all of the protein sequences studied. Myristoylation is a protein posttranslational modification caused by the addition of myristic acid. The amount of myristoylation sites present relates proportionally to the protein's conformational stability for catalytic activity.31 All of the proteins were found to have Microbodies C-terminal targeting signal locations. Phosphorylation sites for protein kinase C and Casein Kinase II were discovered for each protein, except Rv2612c. Proteins with a high number of phosphorylation sites are more likely to be regulated often.32
Protein name |
No of cysteine |
Positions |
Prediction |
Score |
Rv2289 |
4 |
CYS 52 |
Not SS bounded |
-20.8 |
CYS 65 |
Probably no SS bounded |
-9.4 |
||
CYS 155 |
Not SS bounded |
-22.3 |
||
CYS 255 |
Not SS bounded |
-23.1 |
||
Rv2881c |
4 |
CYS 120 |
Not SS bounded |
-29.7 |
CYS 163 |
Not SS bounded |
-29.1 |
||
CYS 181 |
Not SS bounded |
-27.1 |
||
CYS 224 |
Not SS bounded |
-20 |
||
Rv3804c |
3 |
CYS 13 |
SS bounded |
66.8 |
CYS 135 |
SS bounded |
58.5 |
||
CYS 254 |
Not SS bounded |
-46 |
||
Rv1886c |
3 |
CYS 127 |
SS bounded |
72.2 |
CYS 132 |
SS bounded |
64.9 |
||
CYS 251 |
Not SS bounded |
-52.3 |
||
Rv0129c |
1 |
CYS 255 |
Not SS bounded |
-55.5 |
Rv3803c |
No Cysteines |
|||
Rv0564c |
3 |
CYS 26 |
Probably not SS bounded |
-5.5 |
CYS 77 |
Not SS bounded |
-40 |
||
CYS 253 |
Not SS bounded |
-30.6 |
||
Rv2982c |
8 |
CYS 15 |
Probably not SS bounded |
-13.1 |
CYS 159 |
Probably not SS bounded |
-4.6 |
||
CYS 194 |
Not SS bounded |
-35.2 |
||
CYS 202 |
Not SS bounded |
-25.2 |
||
CYS 253 |
Not SS bounded |
-22 |
||
CYS 280 |
Probably not SS bounded |
-11.3 |
||
CYS 289 |
Not SS bounded |
-16.2 |
||
CYS 313 |
Not SS bounded |
-24.5 |
||
Rv2612c |
5 |
CYS 59 |
Not SS bounded |
-37.8 |
CYS 92 |
Probably not SS bounded |
-8.3 |
||
CYS 102 |
Not SS bounded |
-22.7 |
||
CYS 124 |
Not SS bounded |
-28 |
||
CYS 192 |
Not SS bounded |
-23.6 |
||
Rv1822 |
2 |
CYS 149 |
Not SS bounded |
-40.7 |
CYS 160 |
Not SS bounded |
-27.3 |
||
Rv2746c |
1 |
CYS 70 |
Not SS bounded |
-27.3 |
Rv1551 |
1 |
CYS 548 |
Not SS bounded |
-27.3 |
Rv2482c |
1 |
CYS 395 |
Not SS bounded |
-34.6 |
Rv0437c |
4 |
CYS 59 |
Not SS bounded |
-29.5 |
CYS 86 |
Not SS bounded |
-31.5 |
||
CYS 197 |
Not SS bounded |
-45.5 |
||
CYS 230 |
Not SS bounded |
-20.7 |
||
Rv0436c |
4 |
CYS 26 |
Not SS bounded |
-34.4 |
CYS 112 |
Not SS bounded |
-32.6 |
||
CYS 210 |
Not SS bounded |
-32.4 |
||
CYS 231 |
Not SS bounded |
-39.7 |
||
Rv0045c |
No Cysteines |
|||
Rv0914c |
4 |
CYS 78 |
Not SS bounded |
-21.1 |
CYS 93 |
Not SS bounded |
-18.7 |
||
CYS 221 |
Not SS bounded |
-29.3 |
||
CYS 309 |
Not SS bounded |
-39.4 |
||
Rv1543 |
2 |
CYS 112 |
Not SS bounded |
-31.8 |
CYS 218 |
Not SS bounded |
-32.7 |
||
Rv1627c |
No Cysteines |
|||
Rv1814 |
2 |
CYS 11 |
Not SS bounded |
-47.5 |
CYS 165 |
Not SS bounded |
-27.8 |
||
Rv1867 |
2 |
CYS 10 |
Not SS bounded |
-23.5 |
CYS 321 |
Not SS bounded |
-19.5 |
||
Rv2261c |
2 |
CYS 83 |
Not SS bounded |
-27.1 |
CYS 137 |
Not SS bounded |
-15.4 |
||
Rv2262c |
7 |
CYS 15 |
Not SS bounded |
-19.1 |
CYS 114 |
Not SS bounded |
-29.9 |
||
CYS 185 |
Not SS bounded |
-16.6 |
||
CYS 197 |
Probably not SS bounded |
-8.9 |
||
CYS 201 |
Probably not SS bounded |
-13.8 |
||
CYS 332 |
Probably not SS bounded |
-12.5 |
||
CYS 358 |
Not SS bounded |
-18 |
||
Rv3523 |
3 |
CYS 207 |
Probably not SS bounded |
-29.9 |
CYS 214 |
Not SS bounded |
-16.6 |
||
CYS 386 |
Not SS bounded |
-8.9 |
||
Rv3720 |
4 |
CYS 162 |
Probably not SS bounded |
-6.1 |
CYS 200 |
Not SS bounded |
-54.5 |
||
CYS 295 |
Probably not SS bounded |
-10.8 |
||
|
|
CYS 356 |
Not SS bounded |
-34.5 |
Table 2 Number of cysteine residues, their position and disulfide bridge predicted using cys_rec tool
Human and gut flora non-homology analysis
Potential pharmaceutical targets that are homologous to the host could have a negative impact on host metabolism. As a result, finding non-homologous proteins is a critical step in predicting therapeutic targets. Non-homology analysis was performed on the four critical genes, and they were all shown to be nonhomologous to the human proteome.33 Gut microflora plays an important role in metabolism by digesting food particles and preventing dangerous bacteria from colonising the intestine.34 The human host's interaction with gut microbiota is critical for absorbing poorly digested dietary components, xenobiotic breakdown, and vitamin production.35 They also confer resistance to opportunistic bacteria and pathogen colonisation.36 When the healthy gut microbiota population deteriorates, the body's first line of defence against invading microorganisms suffers. It may also result in nutritional deficiencies in the host.37 Following the homology search, all proteins that were non-homologous to gut flora were shortlisted.
Homology modelling
SWISS-MODEL and PHYRE2 SERVER calculated the sequence's 3D structures. Q-mean, Z-score, and Ramachandran plot analysis were performed to evaluate the predicted models. Each server selects the best modelling template depending on how similar the query sequences are. The tertiary structures of the proteins under research were investigated. SWISS-MODEL used the Z-score and Q-mean score, which should be between -4 and +4, to predict successful models for 8 proteins. PHYRE utilised the same criterion to identify models for 5 proteins. SWISSMODEL outperformed PHYRE, according to the aforementioned statistical data. The Ramachandran plot, which was employed in further investigation of the approved models, revealed that 91.875% of amino acids were in the most favourable zone, while 0.34% were in the prohibited zone. Tertiary structures are considered high grade if 90% of the residues are in the chosen region.38
Structure validation
A protein's secondary structure is determined by the pattern of hydrogen bonding, with alpha helices and beta sheets being the most essential types of secondary structures. Alpha-helices are typically seen in water-soluble globular proteins. Protein interactions with other proteins, lipid connections in the cell membrane, and nucleic acid interactions are all common uses for alpha helices. Pharmaceuticals have made use of highly stable alpha helices.39 The amino acid change seen in alpha helix is mostly connected with protein thermostability. Rv2612c contains a larger proportion of alpha - helix (65.44%). (Table 4) Random coils are regions that have an uneven secondary structure but lack hydrogen bonding. Protein enzymatic turnover, flexibility, and structural changes are all dependent on it.40 They can have lengths ranging from 4 to 20 residues. It also enhances protein flexibility during enzymatic turnover, for example. They can withstand mutations better than other structures. With a score of 47.69%, Rv2289 has the largest proportion of random coil, while Rv0129c has a value of 47.65%. The build-up of secondary structures that directly interact with atoms in the main chain results in the formation of extended secondary structures (ESS). It helps to stabilise the original secondary structure as well as future tertiary structures. The largest percentage of ESS (29.87%) is found in Rv0437.41 The SOSUI tool determined that the proteins were 100% soluble. Table 4 illustrates which proteins are soluble.
Protein |
Alpha helix |
Extended strand |
Beta turn |
Random coil |
Solubility |
Rv2289 |
36.15% |
14.23% |
1.29% |
47.69% |
Soluble |
Rv2881c |
47.71% |
14.38% |
7.84% |
30.07% |
Soluble |
Rv3804c |
37.87% |
13.31% |
7.40% |
41.42% |
Soluble |
Rv1886c |
37.54% |
15.69% |
8.62% |
38.15% |
Soluble |
Rv0129c |
34.71% |
11.47% |
6.18% |
47.65% |
Soluble |
Rv3803c |
34.78% |
15.38% |
10.70% |
39.13% |
Soluble |
Rv0564c |
48.68% |
13.78% |
7.92% |
29.62% |
Soluble |
Rv2982c |
51.50% |
11.08% |
6.59% |
30.84% |
Soluble |
Rv2612c |
65.44% |
9.68% |
6.45% |
18.43% |
Soluble |
Rv1822 |
58.37% |
11.00% |
2.87% |
27.75% |
Soluble |
Rv2746c |
57.89% |
11.96% |
4.78% |
25.36% |
Soluble |
Rv1551 |
60.23% |
8.21% |
6.67% |
26.89% |
Soluble |
Rv2482c |
56.53% |
10.39% |
4.56% |
28.52% |
Soluble |
Rv0437c |
24.24% |
29.87% |
6.49% |
39.39% |
Soluble |
Rv0436c |
54.90% |
8.39% |
4.20% |
32.52% |
Soluble |
Rv0045c |
41.28% |
18.12% |
5.03% |
35.57% |
Soluble |
Rv0914c |
43.45% |
11.41% |
7.04% |
38.11% |
Soluble |
Rv1543 |
52.49% |
10.85% |
6.74% |
29.91% |
Soluble |
Rv1627c |
37.06% |
12.44% |
6.97% |
43.53% |
Soluble |
Rv1814 |
45.33% |
17.33% |
3.33% |
34.00% |
Soluble |
Rv1867 |
36.84% |
15.38% |
6.48% |
41.30% |
Soluble |
Rv2261c |
32.14% |
21.43% |
7.14% |
39.29% |
Soluble |
Rv2262c |
41.39% |
17.22% |
6.39% |
35.00% |
Soluble |
Rv3523 |
41.62% |
11.42% |
5.84% |
41.12% |
Soluble |
Rv3720 |
47.14% |
13.81% |
5.95% |
33.10% |
Soluble |
Table 4 Predicted secondary structure elements using SOPMA Software
Molecular docking
A simulation technique known as molecular docking studies the best way to attach a ligand to an active site on a target. This method selects the binding site in the target using 3D coordinates, and the binding affinity of the resulting orientation of the molecule within the binding site, which forms the complex, is determined.42 The complex created when the involved ligand effectively binds with the active pockets of the target has the largest magnitude negative number (highest binding affinity or lowest binding energy).5 bioavailable ligands were used in molecular docking with Mycobacterium tuberculosis Rv2746c and Rv2881c lipid synthesis proteins. The binding energies of these ligands for proteins Rv2746c and Rv2881c are shown in Tables 5 & 6, indicating that they have a high affinity for the target proteins. Complexes for Rv2746c protein exhibited binding affinities ranging from 6.8 to 8.1 kcal/mol, whereas complexes for Rv2881c protein had binding affinities ranging from -7.4 to -9.1 kcal/mol. In lower-binding-energy ligands, additional hydroxyl groups may establish hydrogen bonds with the target protein, indicating a favourable interaction. The pi-sigma bond stabilises the ligand, allowing it to intercalate into the binding sites of the receptor. Alkyl and pi-alkyl linkages also aid ligands in engaging hydrophobically in the binding pocket of the receptor.
Sr.No. |
Ligand |
Binding Energy n(ΔG) (kcal/mol) |
1 |
Genistein |
-7.8 |
2 |
Coumestrol |
-8.1 |
3 |
Daidzein |
-7.1 |
4 |
Nyasol |
-6.8 |
5 |
(Z)-hinokiresinol |
-7 |
Table 5 Binding energy of Rv2746c protein with the selected 5 ligands
Sr.No. |
Ligand |
Binding energy n(ΔG) (kcal/mol) |
1 |
Genistein |
-8.7 |
2 |
Coumestrol |
-9.1 |
3 |
Daidzein |
-8.5 |
4 |
Nyasol |
-7.4 |
5 |
(Z)-hinokiresinol |
-7.9 |
Table 6 Binding energy of Rv2881c protein with the selected 5 ligands
Genistein, a flavonoid, had a binding energy of 7.8 kcal/mol with Rv2746c protein and exhibited a variety of interactions, including Conventional hydrogen bonds with THR153 and ARG134 residues, Carbon Hydrogen bonds with LYS150, Pi-Sigma bonds with ASP73 and ASP94, Pi-Alkyl bond, and Unfavourable Donor-Donor bond with PRO95, as shown in Figure 1. Protein Rv2881c has a comparable binding score of -8.7kcal/mol. Figure 2 depicts numerous interactions, including a SER214 Conventional hydrogen bond, a Carbon hydrogen bond with ASP190 and LYS213, an Amide-Pi stacking bond with GLY193 and GLY197, and an Alkyl and Pi-Alkyl bond with LYS212 and LYS213. This synthetic antibiotic family was shown to have a variety of anti-infective activities, including antiviral action. An in-silico investigation of DHFR protein interactions found that Genistein could interact with and potentially inhibit important Dihydrofolate reductase proteins with a binding affinity of 7.75kcal/mol, which is similar to the data obtained in the current study [genstin].
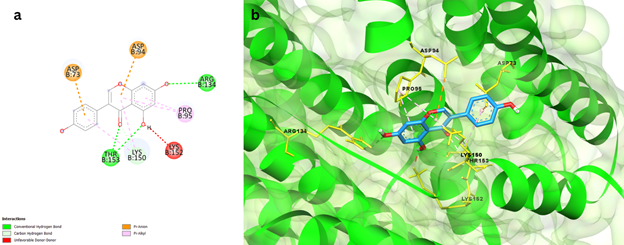
Figure 1 a) 2D interaction plot of Genistein docked in the binding pockets of Rv2746c protein, b) 3D representation showing the position of Genistein within the binding site of Rv2746c protein.
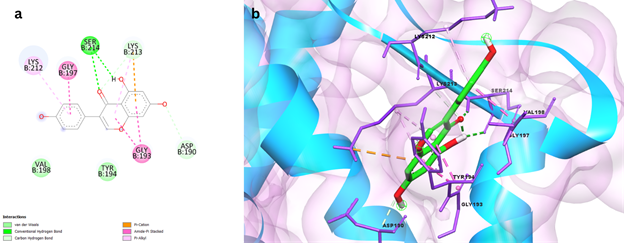
Figure 2 a) 2D interaction plot of Genistein docked in the binding pockets of Rv2881c protein b) 3D representation showing the position of Genistein within the binding site of Rv2881c protein.
Coumestrol docked with Rv2746c protein exhibits a binding affinity of -8.1 kcal/mol, From Figure 3 it can be noted that Coumestrol demonstrated Convention hydrogen bond with THR153, ARG134, LYS99, Pi- Anion bonds with ASP73, ASP94, ASP98 residue and Pi- alkyl Stacked bond with PRO95. Further, it has also showed Pi- Sigma bonds with LYS150 residues. A binding affinity of -9.1 kcal/mol was reported when Coumestrol was docked against Rv2881c protein. As depicted in Figure 4, the ligand Coumestrol established Conventional hydrogen bond with SER214 and ASP190 residue, Amide - Pi stacking bond with GLY193 and Pi- Alkyl bonds with LYS212 residue. LYS213 residue forms a pi-cation link. Zafar et al. used in silico techniques to investigate the influence of structural stability on selective binding of coumestrol to ER-alpha and not ER-beta.
Over the last decade or so, there has been a renaissance of interest in Tuberculosis medicine research, which has resulted in some important scientific discoveries. The current TB therapeutic pipeline includes novel chemical scaffolds and a diverse set of targets. Despite these advances in chemotherapy, TB eradication remains a global concern. As a result, in addition to the previously mentioned scientific endeavours, it is crucial to uncover additional TB targets that are not only important during host infection but also vulnerable to pharmacological inhibition. Tuberculosis is a potentially lethal disease caused by Mycobacterium tuberculosis strains. This bacteria heavily relies on transport through the cell membrane for a variety of biological processes. As previously shown, triacylglycerol are required for many biological activities, including Mtb survival. The purpose of this study was to provide thorough information about proteins involved in lipid synthesis pathways. This network has around 25 such proteins. Using several online tools, physiochemical metrics such as molecular weight, instability index, GRAVY, and so on were computed, and functional motifs and 3D structural information were collected, which may provide light on the biological function of the proteins. Proteins Rv2746c and Rv2881c provided the best qualifying findings based on several physiochemical and biological properties such as structural and functional studies. Molecular docking of these two proteins was also performed with five selected ligands and medicines currently used to treat tuberculosis. When compared to the medications used for therapy, the selected ligands had a remarkable binding energy. A high number of traditional hydrogen bonding was also detected with these two proteins, indicating promising intermolecular protein and drug interactions. Number of cysteine residues, pI, Aliphatic index, Instability Index, GRAVY, and structural folds indicate that these proteins can be chosen as reliable therapeutic targets. In the future, we should identify more plausible and effective therapeutic targets in order to develop new drug candidates with more efficacy.
All data generated or analyzed during this study are included in this published article.
Ethical statement
No animals were harmed during this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.

©2022 Jha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.