Journal of
eISSN: 2376-0060


Research Article Volume 3 Issue 6
PGIMS Rohtak, India
Correspondence: Brijesh Prfajapat, PGIMS Rohtak, A-38 ganesh nagar pandav nagar complex Delhi-92, India, Tel 8607731947
Received: July 04, 2016 | Published: December 15, 2016
Citation: Prfajapat B. Role of mid thigh cross sectional area by computed tomography (MMTCSA CT ), C-reactive protein to assess effect of pulmonary rehabilitation (PR) in interstitial lung diseases (ILDs). J Lung Pulm Respir Res. 2016;3(6):164–168. DOI: 10.15406/jlprr.2016.03.00105
ILDs are a diverse group of lung diseases that are characterized by chronic inflammation and progressive fibrosis of the pulmonary interstitium.1 Data on the prevalence of ILD is scattered, scanty and inaccurate too. The prevalence in New Mexico was 80.9 cases per 100,000 population for men and 62.2 cases per 100,000 population for women and the incidence of ILD was 31.5 per 100,000 persons per year for men and 26.1 per 100,000 persons per year for women with idiopathic pulmonary fibrosis (IPF) accounted for 46.2% of all ILDs diagnosed in men, and 44.2% in women.2
Patients with ILDs often report decreases in exercise tolerance, activities of daily living, muscle force, health-related quality of life (HRQOL), and increases in levels of fatigue and dyspnoea3 and those with the greatest exercise limitation have the worst quality of life.4 The mechanisms of reduced exercise capacity in ILD appear to be multifactorial. Destruction of the pulmonary capillary bed results in ventilation-perfusion mismatch, impairment of gaseous exchange and oxygen diffusion limitation.5 Circulatory limitation aggravates the problem which results from pulmonary capillary destruction and pulmonary vasoconstriction ultimately leading to pulmonary hypertension and cardiac dysfunction in some patients.6 Ventilatory limitation to exercise7 and peripheral muscle dysfunction also play a significant role in limiting exercise capacity,8 as a result of physical deconditioning. Patients who experience dyspnoea and fatigue with functional activity commonly reduce their activity levels, leading to a vicious cycle of worsening exercise capacity and increasing symptoms9 Systemic inflammation may also leads to muscle wasting and peripheral muscle dysfunction like in COPD.10 Cytokines play an important role in the pathogenesis of ILD and several Inflammatory mediators have been shown to be increased in ILDs which include CRP, IL-8, IL-10, TNF-α, IFN-gamma, IL-2, MMP-9, MMP-7, etc. Moreover, treatments for ILD such as corticosteroids and immunosuppressive therapy further may lead to drug induced myopathy.9
Pulmonary rehabilitation (PR) program is a well-established and widely accepted therapeutic tool used with standard pharmacotherapy alleviates symptoms, improves the quality of life and, functional capacity in patients with chronic lung diseases like COPD, bronchiectasis and, thus optimize a patient’s physical and psychological functioning.11 Exercise training is the cornerstone of pulmonary rehabilitation and is the best available means of improving muscle function in chronic lung disease. However, very little is known about the effect of pulmonary rehabilitation over systemic inflammation, muscle mass and exercise capacity in patients of ILD. Holland et al.12 demonstrated short term improvement in exercise capacity and symptoms in ILD patients after 8 weeks of supervised exercise training. Ferreira et al.13 showed a clinically significant improvement in both functional status and dyspnea after 6 to 8weeks of outpatient PR. This benefit was more pronounced in the patients with the worst baseline walk distances. Nishiyama et al.14 showed 10-week programme of exercise training resulted in marked improvement in both exercise capacity and health related quality of life in patients of IPF. Meta-analysis of the studies on physical training for interstitial lung diseases by Holland et al.9 demonstrated improvement in functional exercise capacity, dyspnoea and quality of life immediately following training. However, there was little evidence regarding long-term effects of physical training. Salhi et al.15 showed that pulmonary rehabilitation in patients with restrictive lung diseases leads to clinically relevant improvements of the maximal and submaximal exercise capacity, muscle force, and quality of life after 12weeks and with further improvements after 24weeks with just 16% drop-out i.e half the dropout rate in COPD, whose drop-out rate may reach up to 31%.
Midthigh muscle cross-sectional area (MTCSA) is an index of muscle mass which has frequently been studied in stable COPD patients. Prediction of MTCSA from anthropometric parameters is not sufficiently accurate for clinical purposes in patients with COPD. Marquis et al.16 used MTCSACT in their study and found that it is a better predictor of mortality than body mass index and MTCSACT has a strong impact on mortality in COPD patients with an FEV1<50% predicted. Fiatarone et al.17 found a significant increase in mid-thigh muscle area with marked increases in both quadriceps (9%) and hamstring and adductor areas (8.4%) in response to 8wk of resistance training, without changes in subcutaneous or intramuscular adipose tissue. Recently Ferrari et al.18 showed inverse relationship between inflammatory markers and muscle mass using MTCSACT.
We hypothesize that PR in ILD patients leads to increase in muscle mass as calculated by MTCSACT scan ,decrease in systemic inflammation and improvement in exercise capacity (6MWD) and quality of life (St. George respiratory questionnaire).
The study was a prospective nonrandomized study conducted at Vishwanathan Chest Hospital, Vallabhbhai Patel Chest Institute (VPCI), University of Delhi. Both male and female patients diagnosed as ILD were included in the study from the outpatient department. The diagnosis of ILD was based on clinical history consistent with ILD, chest xray, pulmonary function test, high resolution CT scan (HRCT), broncheoalveolar lavage (BAL) and transbronchial lung biopy (TBLB).
Inclusion criteria were age more than 18yrs and history, examination, PFT consistent with ILD. Exclusion criteria were pregnancy, physiological impairment impeding traing program, presence of systemic disease, acute respiratory infection in the last 4weeks. The baseline values of the following parameters were measured at the time of inclusion in to the study: Complete pulmonary function test (PFT), Mid thigh cross sectional area CT (MTCSACT) (mm2), Six minute walk distance (6MWD) and SGRQ. The patients then received standard therapy as per BTS guidelinies19 in addition to pulmonary rehabilitation for eight weeks and the variables were again measured at the end of eight weeks of PR.
Exit from the Study
Pulmonary function tests
Spirometry was performed on a computerized apparatus- Benchmark (P. K. Morgan and Co. Ltd. Chatham, Kent England). FVC, FEV1, FEV1/FVC%, RV, TLC, DLCO were obtained as per the recommendations of the American Thoracic Society.20
Midthigh muscle cross-sectional area (MTCSACT)
A computed tomography of the right and left thigh, halfway between the pubic symphysis and the inferior condyle of the femur was performed using a third generation scanner. Each image was 10-mm thick and was taken at 120KV and 200mA with a scanning time of 1 second while the subject was lying in the supine position. The thigh muscle cross-sectional area (CSA) was obtained by measuring the surface area of the tissue with a density of 40 to 100 hounsfield units (HU). The MTCSACT was calculated by taking average for right and left thighs.
Six minute walk test (6mwt)
6MWT was performed on a flat, straight, enclosed corridor with a hard surface as per the ATS guidelines.21 Supplemental oxygen was used during the test in patients who were already on LTOT or in those who desaturated below 88%.
Pulmonary rehabilitation program
The pulmonary rehabilitation program comprised minimum of 90minutes of supervised exercise training for lower and upper limbs, performed over separate sessions each day, three days a week, for 8 weeks. Lower limbs training included leg-ergometry and treadmill walking. Training of the upper limbs included arm-ergometry and free weights. Simultaneous upper and lower limb training was performed on Semi-Recumbent Whole Body Exerciser. Exercise intensity during each session was incremental and graded according to symptom tolerance and was of 20minutes duration. Patients also attended educational sessions on topics such as breathing exercises, energy conservation, lung health, medications and stress management.11
Statistical analysis was done using Graph pad 5.03 version. The data were presented as mean±standard error (SE). The difference in the mean baseline values of various measurements within the group and between the groups was made using student’s t-test. A p value of <0.05 was considered significant.
21 patients were enrolled. Eighteen patients completed the study and three patients dropped out as they refused for PR programme after 1week. 11 were females and the mean age was 52.17±2.416years. The demographic and clinical characteristics of the study group is given in the Table 1.
Parameters |
Study Group |
Mean ± S.E |
|
Age (Years) |
52.17 ± 2.416 |
BMI (kg/m2) |
26.31 ± 1.066 |
Male: Female |
7:11 |
Occupation |
Housewife (11) 61.11% |
Shop keeper(2) 11.1% |
|
Clerk (1) 5.55% |
|
Farmer (1) 5.55% |
|
Retired bank officer (2) 11.11% |
|
Tutor (1) 5.55% |
|
Smoking Status |
Smoker (6) 33.33% |
Nonsmoker (12) 66.66% |
|
Pack Years |
5.000 ± 2.425 |
Disease Duration (yrs) |
4.250 ± 0.4753 |
Hb (g/dl) |
12.94 ± 0.3511 |
Total Protiens (gm/dl) |
5.466 ± 0.2255 |
Albumin (gm/dl) |
3.068 ± 0.1846 |
Table 1 Demographic Characteristics of Study Group
Decrease in CRP mean value at the end of 8weeks was found as compared to baseline level although the difference didn’t reach the mark of statistically significant (p=0.08) (Table 2), (Figure 1). MTCSACT showed statistically significant increase at the end of 8weeks compared to baseline level (p=0.0089) (Table 2), (Figure 2). At the end of 8weeks, six minute walk test showed statistically significant improvement from baseline in the study group (p<0.0001) (Figure 3). SGRQ score was also improved post PR (p<0.0001) (Figure 4). Arterial blood gas parameters, blood lactate levels and pulmonary function tests did not show significant change at the end of 8 week. Comparison of various parameters done pre and post PR programme are summarized in Table 2.
Parameters |
At Base Line |
AT 8 Weeks |
P value |
Mean ± S.E. |
Mean ± S.E |
||
CRP (mg/L) |
5.794 ± 0.8470 |
5.286 ± 0.7400 |
p= 0.0838 |
MTCSACT (mm2) |
9336 ± 464.6 |
9696 ± 463.5 |
p= 0.0089 |
6MWT (m) |
411.6 ± 15.27 |
457.9 ± 14.06 |
p < 0.0001 |
Lactate (mmol/L) |
1.156 ± 0.1181 |
1.011 ± 0.1174 |
p= 0.2067 |
SGRQ |
58.51± 1.505 |
52.68 ± 1.659 |
P<0.0001 |
Pao2 (mmHg) |
63.72 ± 2.148 |
64.17 ± 1.905 |
p= 0.4395 |
Paco2 (mmHg) |
39.32 ± 1.183 |
39.27 ± 0.8807 |
p= 0.9631 |
Sao2 (%) |
91.84 ± 1.241 |
92.56 ± 1.155 |
p= 0.1277 |
FEV1 (L) |
1.553 ± 0.1189 |
1.577 ± 0.1204 |
p= 0.3905 |
FVC (L) |
1.859 ± 0.1414 |
1.894 ± 0.1422 |
p= 0.3905 |
RV(L) |
1.066 ± 0.09290 |
1.154 ± 0.1056 |
p= 0.1726 |
TLC(L) |
2.889 ± 0.1581 |
3.125 ± 0.1951 |
p= 0.0600 |
DLCO(ml/min/mmHg) |
9.246 ± 1.025 |
9.819 ± 1.004 |
p= 0.1726 |
Table 2 Comparison of Various Parameters in Study Group (N= 18)
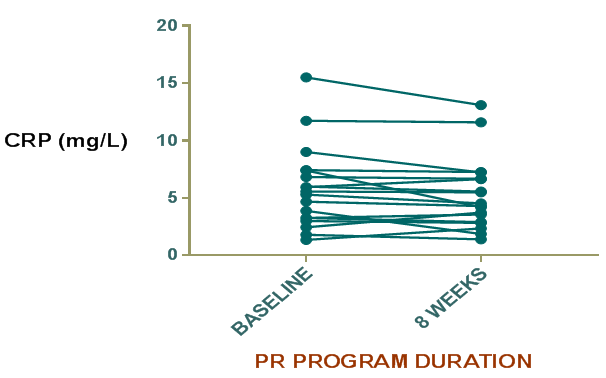
Figure 1 Difference in C-rective protein (CRP) level in the study group before and after PR program.

Figure 2 Difference in Mid thigh cross sectional area using CT scan (MTCSACT) in the study group before and after PR program (p= 0.0089).
We studied the effects of pulmonary rehabilitation on systemic inflammation (CRP levels), MTCSA using CT scan, exercise capacity and quality of life.
CRP reflects total systemic burden of inflammation of individuals and is used as a predictor of hospitalization and mortality in patients with chronic respiratory failure. It is higher in patients in patients with poor FEV1 value and in those who smoke. CRP level predicts cardiovascular mortality. Drent and coworkers demonstarted that a moderate increase in serum CRP is implicated in Sarcoidosis22 while Richards et al.22 found higher serum levels of CRP in myositis associated ILDs.23 In the present study, CRP levels were higher in the study group supporting the results of previous studies. Mattusch and colleagues investigated the influence of exercise training on CRP level in healthy subjects and found that the baseline CRP level in 10 out of 12 runners was significantly reduced after training.24 This study therefore supports the view that intensive regular exercise has a systemic anti-inflammatory effect in healthy subjects and the research has potential implications for patients with ILDs. HERITAGE Family Study of exercise in healthy sedentary individuals also suggested that beneficial reductions in CRP levels after training are greatest in those with baseline levels higher than 3mg/L.25 In our study group mean CRP level in at baseline was high (5.794±0.8470mg/l). In addition, we found CRP levels were decreased, although not significantly after 8 weeks of PR program. Apart from exercise, the medications that can decrease CRP level include inhaled and systemic corticosteroids. The patients in our study receiving pharmacotherapy in the form of corticosteroids and other immunosuppressive drugs were given the same dose of drugs throughout the study period. The decrease in mean value in might be due to the absence of infection and exacerbation during the entire study period which are known to increase the CRP levels. Although the decrease in mean CRP level was not statistically significant still it indicates that pulmonary rehabilitation may have additive effect on the decrease in CRP level. This will provide a promising aspect for further research and highlights the importance of pulmonary rehabilitation in the management of ILD.
Body weight loss is seen in patients with ILD. Although body weight is a useful prognosis marker in ILD, it is not sensitive to changes in body composition as it can be increased or normal despite the presence of muscle wasting. Marquis et al used MTCSACT in their study and found that MTCSACT is a better predictor of mortality than body mass index and MTCSACT has a strong impact on mortality in COPD patients with an FEV1<50% predicted.16 Fiatarone et al.17 found a significant increase in mid-thigh muscle area with marked increases in both quadriceps (9%) and hamstring and adductor areas (8.4%) in response to 8wk of resistance training, without changes in subcutaneous or intramuscular adipose tissue. In our study, the mean value of MTCSA at the end of 8 weeks was increased to 9696±463.5mm2. The increase in mean value from baseline was clinically significant (p=0.0089). Ferrari et al showed an inverse relationship between systemic inflammation and MTCSACT in stable COPD patient.18 In this study, we too found that MTCSACT increased while CRP decreased after PR program.
To the best of our knowledge, no follow up study has been done to see the change in MTCSACT after pulmonary rehabilitation in ILD patients. Muscle wasting should be considered as a serious complication in ILD and other chronic illnesses with important implications for survival. Gain in muscle mass and strength seems to be associated with better exercise tolerance and survival of ILD patients. Thus, improving peripheral muscle function could be a reasonable therapeutic target in these groups.
6MWT is used to assess the functional capacity in ILD and other chronic respiratory diseases patients. This minimum clinically important distance (MCID) of 54m is based on the cross-sectional study of Redelmeir on 112 COPD patients attending a residential pulmonary rehabilitation program.26 Lederer et al.27 showed that the lower 6MWD is strongly and independently associated with an increased mortality rate for wait-listed patients classified as having IPF for lung transplantation and 6MWD was a better predictor of death at 6 month than was FVC % predicted.
Holland28 showed that small differences in six-minute walk distance (6MWD), in the range 29–34m, may be clinically significant for people with diffuse parenchymal lung disease. In our study, the mean value of 6MWD was 411.6±15.27m. The mean value of 6MWD at the end of PR program was increased to 457.9±14.6m compared to baseline (p<0.0001). The impairment of exercise capacity is a central issue in patients with ILD patients. In clinical practice, the 6-minute walk test (6MWT) and the incremental shuttle walking test are commonly used to assess changes in functional exercise capacity following pulmonary rehabilitation with the primary outcome reported being the distance walked during the test. The 6MWT is also be used as a one-time measure of functional status of patients, as well as a predictor of morbidity and mortality in ILD patients.27
In the present study there was a statistically significant reduction in SGRQ score after PR program of 8 weeks.
The mean total score before PR program was (58.51±1.505) and after PR program was (52.68±1.659) with (P<0.0001). The results supported the findings of previous studies. Nishiyama et al.14 also found a statistically significant improvement in the total score of SGRQ score in rehabilitated IPF group than the control group with (P<0.01). Jastrzebski et al.29 found a statistically significant improvement in daily physical activities and in HRQL measured by SF-36 and SGRQ after 12weeks of PR program. Patients with advanced lung fibrosis may be too dyspneic to leave the house or to walk suggesting a clear lack of regular daily physical activities in these patients. Reduced daily physical activity may lead to loss of physical fitness and reduced quality of life seen in patients with chronic lung disease.29,30
Our study has some limitations. First, the study sample was small and thus a large sample size is required to establish the firm role of PR in ILD patients. Second, it was nonrandomized trial and thus a randomized trial is needed in this direction. Thus, we conclude that pulmonary rehabilitation program with exercise training, upper-limb, trunk, lower limb, respiratory muscle training is highly effective in improving the exercise capacity of patients of ILD compared to patients who receive standard medications only.
None.
The author declares no conflict of interest.

©2016 Prfajapat. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.