Journal of
eISSN: 2376-0060


Case Report Volume 5 Issue 3
1Honorary Consultant Pneumology, Hospital general De Agudos Dr. E Torn
2Chief Division Pneumology, Hospital general De Agudos Dr. E Torn
Correspondence: Darío Raúl Rey, Honorary Consultant Pneumology, Hospital general De Agudos Dr. E Tornú, Buenos Aires, Argentina
Received: May 14, 2018 | Published: June 19, 2018
Citation: Raúl Rey D, González JA. Pulmonary alveolar proteinosis secondary to chronic chlorine occupational inhalation. J Lung Pulm Respir Res. 2018;5(3):100-103. DOI: 10.15406/jlprr.2018.05.00171
We present a 38-year-old patient with a history of two months of dyspnea mMRC II-III, cough, low-grade fever and impregnation syndrome. Clinical and radiologic studies suggested a diagnosis of Pulmonary Alveolar Proteinosis (PAP) confirmed by transbronchial biopsy (TBB) and bronchoalveolar lavage (BAL). PAP is one of the "orphan diseases" of the lung, with very low prevalence and incidence. Three clinical variants are known: Idiopathic, Acquired and Secondary. Due to the nature of our patient’s work setting, we diagnosed occupational PAP secondary to the chronic inhalation of chlorine in a poorly ventilated work environment as well as secondary to inadequate respiratory protection. The patient received one session of Total Pulmonary Lavage (WLL) and he clinically responded and improved.
Keyword: pulmonary, transbronchial biopsy, pulmonary lavage, chronic chlorine, pulmonary alveolar proteinosis
Pulmonary Alveolar Proteinosis (PAP) is one of the "orphan diseases" of the lung, whose peculiarity consists in an intra-alveolar accumulation of a lipo-proteinaceous material related to surfactant, which interferes with gas exchange, presenting with symptoms and signs varying in intensity. Initially described by Rosen, Castleman and Liebow in 1958, its frequency is very low: both Diksen and Ben Dov estimate an incidence in 0.37 cases/100,000 people and prevalence in 3.7/1,000,000 inhabitants.1‒3
Three clinical variants are known: Idiopathic,4 Acquired (or autoimmune) and Secondary, which occur during bacterial infections, HIV, hematological malignancies, inhalation of fumes, organic or inorganic particles, such as cotton or aluminum respectively.5‒14 The diagnosis of PAP secondary to chronic chlorine occupational inhalation is even more unusual and rare, warranting this case report.
Our subject is a 38 years old male with a history of 40 pack-years of tobacco use, as well as alcoholism. He has a history of hypertension. He has been employed as a tannery worker for the last 8 years and he presented with a 2-month history of mMRC II-III associated cough, fevers, night sweats and impregnation syndrome. He was referred to a regional hospital. In the Emergency Room, he was found to have hypoxemia associated with bilateral nodule-like opacities on his Chest X-ray and was admitted. On clinical examination, he was sub-cyanotic with an Oxygen saturation at rest breathing air of 86%. He was found to have "Clubbed" fingers, pulse rate of 88 per minute, blood pressure of 110/60mm Hg. Respiratory examination revealed fine moist rales in middle and lower fields of both lungs.
Studies carried out: Serology for HIV, B and C hepatitis: Negative.
Chest X-ray (CXR): Images with alveolar pattern appearance on both pulmonary fields with predominance of middle and lower zones (Figure 1). A Chest Computed tomography scan of the thorax (CT): showed no mediastinal adenopathies. Extensive alveolar commitment of bilateral and geographic distribution at the level of both lungs with a "crazy paving" type. Ventilated areas at the level of apical and posterior segments of right superior lobe (Figure 2).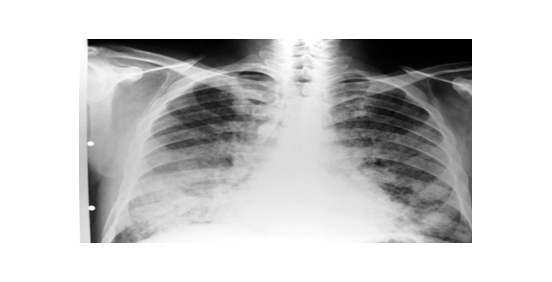
Figure 1 Alveolar pattern appearance on both pulmonary fields with predominance of middle and lower zones.
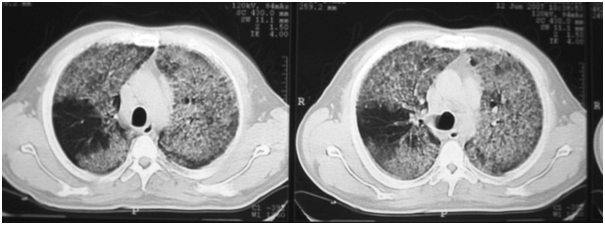
Figure 2 Extensive alveolar commitment of bilateral and geographic distribution at the level of both lungs with a crazy paving type.
Spirometry: revealed FVC 1380ml (31%) FEV1 1240ml (35%) and FEV1/FVC 89% corresponding to a Severe Restrictive Pattern.
Due to a lack of access to advanced pulmonary testing facilities at the treating hospital, neither a DLCO nor titration of Antibodies Anti-Granulocyte-Macrophage Colony-Stimulating Factor (anti GM-CSF), in blood were made.
Test performed included:
The patient gave a more detailed history of his work-related activities over the period of 8 years in tanning, which consisted of immersing raw leathers in a solution with hydrochloric acid, leaving them drying for 2-3 months prior to processing leathers. He performed these work tasks with inadequate respiratory protection on broad, uncovered patches, despite which, he usually presented symptoms of irritation in the upper airways and ocular apparatus (irritative cough and conjunctival inflammation) during and after work. Based on the studies carried out as well as his work history, the case was interpreted as a Secondary Alveolar Proteinosis due to chronic chlorine exposure.
Treatment Intervention: One session of whole lung lavage (WLL) was performed, evolving favorably and improving oxygenation without requiring supplemental oxygen. Laboratory at discharge: GR 6.380.000/mm3 Hto 53% GB 14.900/mm3 Oxygen saturation at rest breathing air 93%. Normal Ionogram. It was decided to be discharged from hospital and periodically examined on External Office, but the patient did not return to control.
Accidental, repeated and chronic chlorine inhalation at low environmental concentrations in the industry, causes irritation of the oropharyngeal and ocular mucous membranes, chronic irritant cough and an influenza-like condition.15 These episodes cause a limitation to airflow and changes in lung function, facts that have been well researched in the specialized literature.16
The surface of the pulmonary alveolus is covered by a thin line of surfactant, composed of 90-95% of lipids and 5-10% of proteins, which reduces surface tension; it prevents collapse and transudation of capillary fluid in the alveolar lumen. The surfactant proteins (A-B-C and D) have properties such as partaking in metabolism, opsonizing pathogenic microorganisms and stimulating the functional capacity of alveolar macrophages.17
In PAP, fundamental finding consists of a defect of the alveolar macrophages to process the pulmonary surfactant, which accumulates in the intra-alveolar amorphous material as well as in the detritus of these cells. Medical knowledge about PAP was intensified when studying homozygous mice, with suppression of the gene producing GM-CSF, developed a PAP-like disease. The administration of aerosolized GM-CSF to the affected mice, reversed the lung lesions.18
PAP can present 3 clinical variants:
As in Sarcoidosis, the clinical course in these patients can be variable and non-specific and present a clinical dissociation in relation to what is seen on CXR and by testing. Progressive dyspnea and unproductive cough usually have an evolution of 6-7 months in 75% of the cases and the clinical findings show inspiratory rales in 50%, cyanosis in 25% and in a very low percentage, the presence of “clubbing fingers”. Weight loss and low-grade fever are not as frequent. In 13% of cases, a respiratory infection can be associated with Nocardiaasteroides.19
The diagnostic studies identified full alveolar compromise located in the middle and lower fields, with predominance in the perihilar regions, which configures a "butterfly wing"- like.20 However, it is CT that provides high diagnostic yield by confirming the existence of alveolar geographic consolidation, with or without air bronchogram, which is associated with "ground glass opacitiy" and thickening of the interlobular and septal septa, giving the appearance known as "crazy paving". Although it is not specific to PAP, as in this case, it may suggest the diagnostic orientation associated with a clinical and functional context.21,22 Functional evaluation of these patients reveals hypoxemia, a restrictive respiratory pattern, a mild decrease in FVC and TLC, as well as a severe decrease in DLCO, directly related to the involvement of the lung parenchyma.
Although the clinical picture as CT suggests the diagnosis, high suspicion is presented before performing a FOB, since the washing liquid presents an opalescent and milky appearance that, on microscopic examination, has foamy macrophages as well as eosinophilic bodies in a background of granular material that is stained with PAS. LDH is elevated in both serum and bronchial lavage.20 Using electron microscopy, amorphous granular waste with osmophilic structures like the so-called lamellar bodies can be observed.
The "gold standard" is the lung biopsy that allows us to appreciate the histological pattern described in our observation, although performing a transbronchial biopsy also has a high yield because it is a diffuse pulmonary disease.23 An interesting fact linked to PAP, is that anti-GM-CSF antibodies were detected in the BAL practiced in the acquired forms, which are found neither in the other variants of the PAP, nor in other pulmonary conditions and, by logic, neither in the normal lung. This finding has allowed the development of a serological test that has a sensitivity of 100% and a specificity of 98% for the diagnosis of the acquired variant of PAP24 Titration of high GM-CSF, correlates with a lower possibility of therapeutic response.25 The therapeutic behavior to be carried out in this infrequent condition depends on its clinical form. In Congenital, the performance of a pulmonary transplant, successfully allowed the restitution of lung function in patients with PAP due to lack of surfactant B protein.24
In Secondary PAP, unilateral total pulmonary lavage (WLL) is performed. This is a traditional physical method that has given good results. Seymour reports that 63% of PAP cases are submitted to WLL, calculating that 20% of them remain free of symptoms after 3 years. It is as safe to perform as bilateral washing, keeping patients free of symptoms for an average of 15 months. This author found a survival of 94% of patients undergoing WLL versus 85% of those who did not undergo this procedure.19 In localized clinical forms of PAP, Cheng advocates the use of FOB with good results.26 Complications that can occur (hypoxemia, pneumonia, distress and pneumothorax) are scarce.19
The WLL is an unregulated procedure and with which the different researchers act with success, although without a non-uniform methodology. Trying to establish such behavior, Campo et al. sent a questionnaire to 79 Centers about the technique used, lung initially chosen, amount of physiological solution used, etc. answering 20 Centers from 14 countries.27 The summary of the results obtained was as follows:
In the Acquired or autoimmune clinical variant, where the presence of anti-GM-CSF antibodies is detected, therapeutic successes have been reported both with the administration of GM-CSF subcutaneously and by an aerosolized form. This therapy is well tolerated and results have been good in both modes of administration,
although cohorts have been small in number. The optimum dose must still be adjusted and the knowledge that its application can cause distant effects.25, 28‒30
PAP is an "orphan disease" whose natural evolution is not completely known. Its pathogenesis has been partially clarified, especially in the acquired or autoimmune form, by the implication of GM-CSF and the presence of anti-GM-CSF antibodies. The published casuistry of around 500 cases according to Ioachimescu, shows both patients with remission and spontaneous remission as with prolonged lapses of quiescence.31 Beccaria in her series of 21 patients undergoing WLL, only had 30% relapses although they persisted with some degree of functional impairment.32 With this therapeutic modality according to Pressneill, the survival at 5 years is calculated at 80%.33‒34
None.
Author declares there is no conflict of interest.

©2018 Raúl, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.