Journal of
eISSN: 2376-0060


Literature Review Volume 7 Issue 1
1Department of Pediatrics and Human Development, Michigan State University, USA
2Department of Physiology, Michigan State University, USA
Correspondence: Bruce D Uhal, Department of Physiology, Michigan State University, 567 Wilson Rd, East Lansing, MI 48823, USA
Received: December 30, 2019 | Published: January 29, 2020
Citation: Mohamed T, Abdul-Hafez A, Gewolb IH, et al. Oxygen injury in neonates: which is worse? hyperoxia, hypoxia, or alternating hyperoxia/hypoxia. J Lung Pulm Respir Res. 2020;7(1):4-13. DOI: 10.15406/jlprr.2020.07.00221
Premature birth results in an increased risk of respiratory distress and often requires oxygen therapy. While the supplemental oxygen has been implicated as a cause of bronchopulmonary dysplasia (BPD), in clinical practice this supplementation usually only occurs after the patient’s oxygen saturation levels have dropped. The effect of hyperoxia on neonates has been extensively studied. However, there is an unanswered fundamental question: which has the most impact- hyperoxia, hypoxia or fluctuating oxygen levels? In this review, we will summarize the reported effect of hypoxia, hyperoxia or a fluctuation of oxygen levels (hypoxia/hyperoxia cycling) in preterm neonates, with special emphasis on the lungs.
Keywords: Bronchopulmonary dysplasia, oxidative stress, renin angiotensin system, lung injury
BPD, bronchopulmonary dysplasia; ROS, reactive oxygen species; HIF, hypoxia inducible factor; RAC, radial alveolar count; AP-1, activator protein-1; AGT, angiotensinogen; RCTs, randomized controlled trials; ACE, angiotensin-converting enzyme
Bronchopulmonary dysplasia (BPD) is a form of chronic lung disease that affects premature newborns and infants and is the result of damage to the lungs caused by mechanical ventilation and long-term use of oxygen.1–4 Despite many advances in neonatal ventilation techniques and the widespread use of surfactant and antenatal corticosteroids, the incidence of BPD has been reported to be relatively stable at approximately 40% of surviving premature infants ≤28 weeks gestational age, with an estimated 10,000–15,000 new cases annually in the US alone.5–11 Mechanical ventilation and excessive oxygen supplementation are well-studied risk factors for BPD.12–14 Both the airway and parenchyma of the lung tissues are affected. These abnormalities have been attributed to ventilator induced injury as well as to oxygen therapy.15
BPD is recognized as a chronic lung disease of infancy that presents as a systemic syndrome and can be associated with neurodevelopmental deficits, cognitive impairments, failure to thrive, pulmonary hypertension and cor pulmonale.16 High rates of in utero and perinatal exposure to infection may be causally related to preterm delivery and subsequent lung injury.17 Over the past three decades, the histological presentation of BPD has changed from heterogeneous pulmonary inflammation and fibrosis (“Old BPD”) to uniform arrest of alveolar development and variable interstitial cellularity and/or fibroproliferation (“New BPD”).18,19
Reactive oxygen species (ROS) generation in the perinatal period
The fetus normally lives in a physiologically hypoxic environment, and relative hyperoxic exposure can cause an increase in the generation of “reactive oxygen species” (ROS). Preterm newborns are particularly vulnerable to oxygen toxicity due to inadequate levels of antioxidant enzymes, and hence to decreased protection from oxidative injury of rapidly growing tissues.20–24 The developing lung of the neonate is a perfect example of those vulnerable tissues; the endothelial cells and the alveolar type II cells are especially susceptible to oxidative injury. Oxidative stress in these cells activates transcription factors and pathways leading to cellular dysfunction, inactivation of surfactant, and impaired cell survival.23–27
Several pre- and post-natal adverse events generate ROS exposure and contribute to BPD pathogenesis; these include hypoxic, hyperoxic, and mechanical stimuli.28 Excess levels of oxygen (hyperoxia), as occur in supplemental oxygen administration, generate accumulation of ROS 1. Hypoxia/hypoxemia, as in episodes of oxygen desaturations , also results in generation of ROS via a superoxide burst that occurs rapidly with hypoxic exposure.29–31 This ROS generation has been shown to be necessary for stabilization of Hypoxia Inducible Factor 1α (HIF-1α) and activation of the HIF system.27,29,30
The ROS generated by both hyperoxia and hypoxia can result in alterations in cellular proliferation and apoptosis. Mechanisms may involve dysregulation of key transcription factors involved in ROS signaling such as HIF, nuclear factor E2 related factor 2 (Nrf2), NF-κB, and activator protein-1 (AP-1).1,2,32 Altered regulation of these transcription factors and pathways can disrupt postnatal alveolar development, cause inflammation, and potentially lead to fibrosis in preterm infants.33,34 ROS generation activates the HIF system.27 Nrf2 deficiency was shown to augment lung injury and arrest of alveolarization caused by hyperoxia during the newborn period.32 Both NF-κB and AP-1 are activated in multiple cell types, as well as in lung, following hyperoxia.2 A summary of ROS generation and neonatal lung injury is summarized in Figure 1.
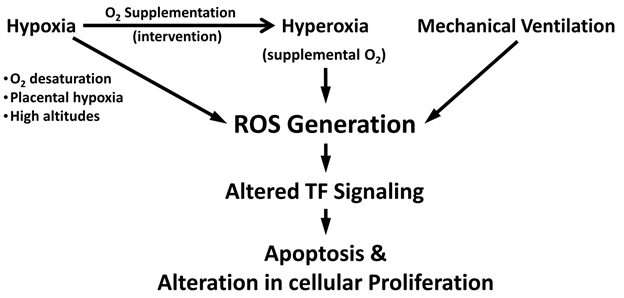
Figure 1 Schematic diagram illustrating reactive oxygen species (ROS) generation in neonatal pulmonary disease.
Prenatal hypoxia
Tissue injury due to oxidative stress is also noted in placental hypoxia, as is seen in preterm born neonates of pre-eclamptic mothers, where ROS are elevated and antioxidant levels are decreased in the maternal circulation.26,35,36 Placental hypoxia also induces an imbalance between pro-angiogenic and anti-angiogenic factors inhibiting VEGF signaling.37,38 This reduction in VEGF signaling in the developing lung was shown to result in impaired pulmonary vascular growth and alveolarization in neonatal rats.39 Maternal exposure to hypobaric hypoxia at high-altitudes can alter placental function, influence oxygen delivery to the fetus, result in lower birth weight, and increase the risk for pre-eclampsia.40,41
Postnatal hypoxia
Premature babies are exposed post-natally to chronic or intermittent hypoxia as a result of immature lungs, apnea of prematurity, inadequate ventilation, or persistence of intrapulmonary arteriovenous shunts causing hypoxemia.27,42 Hypoxia of the infant leads to generalized pulmonary vasoconstriction and increases the pulmonary vascular resistance, causing pulmonary hypertension. Sustained hypoxic pulmonary vasoconstriction causes vascular remodeling of the pulmonary vascular bed leading ultimately to right heart failure.43 Postnatal hypoxia causes impaired alveolarization and alveolar simplification with fewer and larger alveoli. The impaired alveolarization also impairs the vascular maturation in the alveolar wall via mechanisms involving altered signaling of HIF-1α, VEGF, and TGF-β,27,44,45
Postnatal hyperoxic interventions
Preterm birth leads to premature transition of the pulmonary circulation from the physiologic hypoxic fetal environment to a relative hyperoxic postnatal environment (room air). In addition, oxygen supplementation is often required to fulfill the oxygen demands for adequate functioning of the body’s tissues and organs, in particular the brain, intestines, and kidneys.28 systemically circulating oxygen saturation levels (SPO2) is generally targeted to reach above 85%. However, the optimal systemic oxygen saturation in preterm infants is currently not clear. To compensate for the premature lung's simplified alveolar structure and thick alveolar septae, higher levels of supplemental oxygen are often required to achieve the targeted intravascular oxygen levels, further augmenting the already existing (relative) postnatal hyperoxic state.25,27 Hyperoxia leads to impaired VEGF expression and disrupted angiogenesis and alveolarization due to rapid proteasomal degradation of HIF-1α.46–48 This induces vascular arrest, leading to pulmonary vascular diseases. Relative hyperoxia also increases generation of ROS and induces oxidative stress, an important contributor to the development of neonatal BPD. In addition, lower levels of antioxidants including vitamin E, transferrin, and superoxide dismutase, and higher levels of free iron further predispose preterm infants to oxidative injury.20,21,23,49,50
Mechanical ventilation in BPD
Although mechanical ventilation is often essential and life-saving, it can provoke ventilator-induced lung injury in severely premature infants mainly by over-stretching of the distal epithelium and capillary endothelium.25,51–5 The development of that injury is dependent on the developmental stage of the lung, and the type, duration, volume and pressure of the mechanical ventilation.52,54 Mechanical ventilation also results in down regulation of VEGF-1 and its receptor flt-1 and up-regulation of the TGF-β co-receptor endoglin. This imbalance in mechanically ventilated lungs likely contributes to altered alveolarization and angiogenesis.28,55,56
RAS and local tissue injury
The RAS (Figure 2) is traditionally known to play a significant role in blood pressure regulation. Renin is produced by kidney and acts on circulating angiotensinogen (AGT) protein. Renin cleaves AGT to produce angiotensin I (Ang I). Ang I is converted by angiotensin-converting enzyme (ACE) to Ang II, which exerts its actions through binding to specific cell surface angiotensin receptors. Two main receptors to Ang II have been identified; AT1 and AT2; both belong to a super family of seven transmembrane G-protein coupled receptors. The AT1 receptor mediates all of the classical actions of Ang II (vasodilatation, sodium retention, cell growth and proliferation), while the AT2 receptor promotes vasodilatation, cell differentiation, inhibition of cell growth and apoptosis and may play a counterbalancing role to the effects of Ang II on the AT1 receptor.57 ACE-2 and its product angiotensin 1–7 (Ang 1-7) acting on its receptor Mas were shown to have counteracting effects against the adverse actions of the other RAS components. Findings from numerous experimental studies have suggested notable protective effects of ANG1-7/Mas activation in the cardiovascular system.58 Local tissue effects of RAS have been identified in a variety of tissues such as heart, kidney, liver, lung, brain, pancreas and adipose tissue, where RAS component expression has been detected.59,60 Local RAS is involved in injury and inflammatory and fibrogenic diseases of many organs including heart,61,62 lung,63–65 liver,66 pancreas,67 and kidneys,68,69 by mechanisms independent of the blood-derived RAS.
The role of RAS in BPD
RAS is believed to play a role in neonatal lung development and BPD pathogenesis (Figure 2). Perinatal exposure of animal models to ACE inhibitors was shown to disrupt normal alveolar and secondary septal formation during lung development in neonatal pups.70 In addition, neonatal rat ACE inhibition lowered the surface tension of bronchoalveolar lavage fluid and caused widening of respiratory airspaces and thinning of alveolar septa.71 Autopsy of human BPD patients showed decreased ACE expression in lungs as compared to controls without lung disease.72 Wagenaar et al.4 reported that Mas receptor and angiotensin receptor 2 (AT2) agonists reduced inflammation of oxygen-induced lung injury in rats. In this study, mRNA levels of the RAS component genes were measured during normal lung development. The mRNA levels of AT1, AT2, and ACE-2 decreased gradually, whereas expression of angiotensinogen and ACE-1 increased gradually as the neonatal rat lung develops. Exposure to 100% oxygen for 10 days resulted in an increase in expression of AT2 and a decrease in expression of AT1, angiotensinogen, and ACE.4 These studies suggest a critical role for angiotensin-angiotensin receptor signaling involving pulmonary alveolarization in the normal physiology of the neonatal lung and in the pathophysiology of BPD,. Angiotensinogen and ACE-1 play a role in alveolar development and septation, while the reported lower expression levels of ACE-2 in the neonatal lung could be facilitating the lung injury in the neonatal period.4,70–72
The protective potential of ACE-2 in BPD
ACE-2 has been shown to play a protective role in lung disease through effects mediated by the receptor Mas, the receptor for the ACE-2 peptide product ANG1-7.3,4,65,73 Previous studies from our lab and other groups suggest that ACE-2 is down-regulated in fibrotic conditions of the adult and neonatal human lung,3,65,73 via Mas receptor mechanisms.74 We have also demonstrated that ACE-2 regulates alveolar epithelial cell survival by balancing the proapoptotic Ang II and its antiapoptotic degradation product Ang 1-7, through Ang 1-7 action on its receptor Mas.74 Furthermore, we showed that ACE-2 is expressed in fetal human lung fibroblasts but is significantly decreased by hyperoxic lung injury in a cell culture model.3 Importantly, this effect was reversed by hypoxia preceding hyperoxia.65 Recombinant human ACE-2 has been tested in healthy individuals in clinical trials to determine medication pharmacokinetics and pharmacodynamics,75 and has been investigated as pipeline drug “GSK2586881” in a pilot clinical trial to treat adult acute lung injury.76 ACE-targeted therapies might be future beneficial treatments for BPD.77
Premature infants are known to experience intermittent episodes of hypoxemia lasting from a few seconds to several minutes 78. Postnatal exposure to intermittent hypoxia followed by interventional hyperoxia induces oxidative stress and free radicals, which leads to direct cellular injury, oxidation of DNA, induction of cytokines, and recruitment of neutrophils and macrophages to the lung, manifested as pulmonary inflammation.20,21 Furthermore, ROS are released by immune cells resulting in epithelial and endothelial cell injury.26 Clinically unsuspected oxygen desaturation occurs frequently in preterm infants with and without bronchopulmonary dysplasia, and profound hypoxemia is claimed responsible for sudden unexplained deaths in these infants.79 Furthermore, infants who develop BPD experience more frequent episodes of oxygen desaturations than infants who recover from respiratory distress syndrome without developing BPD.19,80
Very few studies in literature directly compare the effects of hyperoxia, hypoxia, or their fluctuation on the neonatal lung development or injury. Table 1 summarizes the studies discussed below. Ratner et al.19 demonstrated that cycling hypoxia with hyperoxia episodes exacerbated lung injury in neonatal mice.19 In their study they tested the effects of hypoxic episodes on a normoxia background, continuous hyperoxia, and hypoxic episodes combined with a hyperoxia background on neonatal mice and compared the results with the normoxia group. Compared with the normoxia control, the hypoxic episodes on normoxia background had no significantchanges on radial alveolar count (RAC) as a marker of lung injury, on oxidized glutathione, or on protein carbonyls as markers of oxidative stress, while continuous hyperoxia significantly reduced RAC and increased oxidative stress markers compared with normoxia. Interestingly, the intermittent hypoxia on hyperoxia background group of mice showed significantly lower RAC and higher oxidative stress markers compared with continuous hyperoxia. Their study suggested that the combination of two oxidative stress mechanisms, hypoxia and hyperoxia, cause a more profound lung injury than either one alone. Furthermore, their study even suggests that hypoxic mice did not have any BPD-like changes.19
Reference |
Organism |
Cycling Conditions |
Cycling Effect |
|
hypoxia |
HYPEROXIA |
|||
Ratner et al.19 |
Mouse, start at P3 |
8% O2 (N2 balanced) |
65% O2 |
Fewer alveoli compared to HYPEROXIA |
|
|
Total 4 Weeks |
||
Schmie dl etal.81 |
Mouse, start with pregnant mothers E14 |
10% O2 |
75% O2 |
Higher volume of the parenchymal airspaces |
Valencia et al. 82 |
Rat, start at P0 |
12% O2 |
50% O2 |
At P23 and P45, compared with normoxia: |
|
|
Total 2 Weeks (P0->P14) |
||
Chang et al.84 |
Rat, start at P0 (within 5hr of birth) |
12% O2 |
50% O2 |
No HYPEROXIA only group was done |
|
|
Total 2 Weeks (P0-> P14) |
||
Sucre et.al.83 |
3D organoid human fetal lung fibroblasts |
10% O2 |
70% O2 |
Markers of fibroblast activation were increased vs normoxia |
|
|
Total 4 days |
||
Table 1 Summary of animal model studies for hypoxia-Hyperoxia fluctuation (Cycling)
A study by Schmiedl et al.82 on animal models of BPD compared the different effects of prenatal hypoxia and postnatal hyperoxia in neonatal mouse lung. Compared with normoxia controls, the lung volume, total air space volume and total septal surface were significantly reduced in the postnatal hyperoxia groups compared with either prenatal normoxia or prenatal hypoxia. The volume weighted mean volume of the parenchymal airspaces and the wall thickness of septa was significantly higher, and the volume density and the volume weighted mean volume of lamellar bodies in alveolar epithelial cells type II (AEII) were significantly lower in the prenatal hypoxia- postnatal hyperoxia group compared with normoxia controls, while prenatal normoxia-postnatal hyperoxia did not cause these changes. The study suggested that the prenatal hypoxia and postnatal hyperoxia model was found to best reflect morphological changes in lung development comparable with alterations found in BPD.81
A recent study by Valencia et al.82 examined the effects of intravitreal bevacizumab, a treatment for retinopathy of prematurity (ROP), on the lungs of a newborn rat pup model. The study showed long term effects of exposure to intermittent hypoxia and hyperoxia during the first 2 weeks of life. At postnatal days P23 and P45, both pO2 and SaO2 were lower in intermittent hypoxia exposure, while hyperoxia alone increased pO2 compared to the room air group. Interestingly, no significant difference was found in SaO2 in the hyperoxia group compared to room air.82 The results of this study suggest long-term effects of hypoxemia-hyperoxemia fluctuation on blood oxygenation and indicate the presence of a discrepancy between pO2 and SaO2 measures. In a 3-dimiensional cell culture organoid model of BPD, Sucre et al.83 showed that markers of fibroblast activation were increased by hypoxia-hyperoxia cycling as seen in BPD; α-SMA, Col1A1, TGFβ1, and PDE5a, and downstream targets of Wnt signaling (Cyclin D1, MMP2, and MMP9 RNA) were increased in hypoxia-hyperoxia relative to normoxia cultured organoids.83 A study by Chang et al.84 investigated the effects of hyperoxia with intermittent hypoxia on neonatal rats and found that repeated intermittent hypoxia during hyperoxia can alter biomarkers responsible for normal microvascular and alveolar development.84 However, both the Sucre et al.83 and Chang et al.84 studies have not compared the effects of hyperoxia alone to the effects of intermittent hypoxia during hyperoxia.
Several clinical studies and randomized controlled trials (RCTs) were performed to determine the range of optimal saturation by pulse oximetry in preterm infants receiving supplemental oxygen.85–91 Many of these studies have been discussed in detail in other reviews,92,93 we summarize these in Table 2. The assessment outcomes for these studies involved mortality, morbidity, development of chronic lung disease, and ROP. The current 2016 update of the European Consensus Guidelines on the management of neonatal respiratory distress syndrome recommends an oxygen saturation target between 90 and 94% with suggested alarm limits of 89 and 95%.93 These guides are based on the NeOProM meta-analysis study of the three largest and most recent RCTs 94; the Surfactant Positive Pressure and Pulse Oximetry Randomized Trial (SUPPORT),89 the Benefits of Oxygen Saturation Targeting II (BOOST II),90 and the Canadian Oxygen Trial (COT).91 In these trials oxygen saturation monitoring by pulse oximetry was the method used to monitor oxygen levels. Pulse oximetry is currently the prevalent monitoring technology to detect blood oxygenation.95 However, invasive blood gas analysis is needed in order to monitor the oxygenation status when SpO2 is close to saturation (≥97%). A new technological measure using multiwavelength pulse co-oximetry called oxygen reserve index (ORI) can be used to monitor oxygenation in the moderate hyperoxemic range (PaO2 100–200mmHg). The ORI is an index with a unit-less scale between 0.00 and 1.00, which is a relative indicator of changes in PaO2 in the moderate hyperoxemic range, and is used as a companion to pulse oximetry monitoring in patients receiving supplemental oxygen.96
Study (ref) |
High SO2 level |
Low SO2 Level |
Outcome(s) |
Tin and Gupta,85 |
88–98% |
70–90% |
- Decreased incidence of ROP with low SO2 |
- No differences in mortality and morbidity |
|||
- High SO2 had more cognitive disabilities after 10 years |
|||
Oxford Vermont Network,86 |
>95% |
<95% |
- Less chronic lung disease and ROP incidence in low SO2 group |
STOP-ROP,87 |
96–99% |
89–94% |
- No significant difference in the rate of progression to threshold |
- Increased incidence of chronic lung disease and a longer duration of hospitalization with high SO2 |
|||
BOOST I,88 |
95–98% |
91–94% |
- No differences found in growth and neurodevelopmental measures at a corrected age of 12 months |
- In the high SO2 group the newborns required oxygen for a longer period, had a higher dependence on oxygen at 36 weeks of postmenstrual age and needed home oxygen therapy with higher frequency |
|||
SUPPORT,89 |
91–95% |
85–89% |
- No significant differences in severe ROP development, death before discharge from the hospital, or both |
- Lower SO2 resulted in a decrease of occurrence of severe ROP |
|||
- Lower SO2 resulted in an increase of death before the discharge |
|||
BOOST II, 90 |
91–95% |
85–89% |
- Lower SO2 was associated with a higher risk of death and necrotizing enterocolitis |
- Lower SO2 resulted a reduction of incidence of ROP |
|||
COT, 91 |
91–95% |
85–89% |
- No significant differences in death before 18 months of corrected age or survival with one or more disability |
|
|
|
- Lower SO2 resulted in a reduction of duration of O2 therapy |
Table 2 Summary of clinical trials using oxygen saturation in neonatal care
Retinopathy of prematurity
ROP is a disease affecting the development of the retinal vasculature characterized by abnormal growth of retinal blood vessels in the incompletely vascularized retina in preterm infants receiving supplemental oxygen therapy. ROP was first reported by Theodore L. Terry in 1942 and referred to as “retrolental fibroplasia”.97,98 An increase in arterial oxygen saturation, as when the preterm infant is resuscitated with high oxygen concentrations, is believed to be damaging to the newly developed retinal capillaries. After a preterm infant is no longer in supplemental oxygen, the avascular retina becomes hypoxic, leading to overexpression of angiogenic factors and vasoproliferation of intravitreal blood vessels.99–101 Penn et al.102 reported that exposure to variable hyperoxia (hypoxia/hyperoxia fluctuations) has been shown to be much more effective at producing proliferative retinopathy in neonatal rat than exposure to constant hyperoxia.102 Numerous animal studies established that intermittent hypox(em)ia cycling with hyperox(em)ia produces severe oxygen induced retinopathy.82,103–107 These studies emphasize the critical role of oxygen level fluctuations.
Heart
Several independent studies in humans and animal models have reported that chronic fetal hypoxia can trigger a fetal origin of cardiac dysfunction and increase the risk of cardiovascular disease in later life.108–115 A transcriptomic study of neonatal ventricular chamber growth and development during perinatal circulatory transition identified Wnt11 as a prominent regulator of chamber-specific proliferation. Perinatal hypoxia treatment in mice suppressed Wnt11 expression and was associated with cyanotic congenital heart defect (CHD) phenotypes and correlated with O2 saturation levels in hypoxemic infants with Tetralogy of Fallot (TOF).116 Several animal studies have shown cardiovascular adverse effects of neonatal hyperoxia with long-term consequences into adulthood. Mouse model studies on perinatal or maternal inflammation combined with neonatal hyperoxia exposure showed altered fetal development affecting cardiac structure and function, resulting in early cardiac dysfunction leading to cardiac failure in adulthood.117,118 In newborn pigs, hyperoxia was found to trigger oxygen free radical-mediated membrane injury together with an inability of the newborn heart to up-regulate its antioxidant enzyme defenses while impairing myocardial function and hemodynamics.119 In addition, neonatal hyperoxia exposure was shown to increase systemic blood pressure and impair vasoreactivity in adult rats,120 possibly due to developmental programming of endothelial nitric oxide synthase uncoupling and enhanced vascular oxidative stress.121 Neonatal hyperoxia exposure also increased adult airway reactivity and was associated with left ventricular (LV) dysfunction in adult mice.44 Cardiac effects of hyperoxia also affect adults. In adult patients with and without congestive heart failure (CHF), hyperoxia was associated with impairment of cardiac relaxation and increased LV filling pressures. These studies indicate that caution should be used in the administration of high inspired O2 fractions.122
Kidney
Oxygen supplementation and hyperoxic exposure of newborn animal models resulted in enlarged renal corpuscles and decreased number of nephrons in the kidneys in early adulthood.120,123 Neonatal hyperoxia exposure also resulted in impaired nephrogenesis causing reduction in both nephrogenic zone width and glomerular diameter and increased apoptotic cell count.124 Since the kidneys are highly vascular organs, the relative hyperoxia and oxygen supplementation in preterm infants could be responsible for the renal developmental abnormalities and for affecting glomerular vascularization, and should be examined in future studies.
Various groups are shedding light on the importance of events that are commonly seen in neonatal ICUs, particularly with extremely low birth weight preterm babies, and with how prematurity affects the lung as well as other organs. Hyperoxia might not be the only risk factor for worsening BPD. However, uncontrollable fluctuation/cycling between hypoxemia, normoxemia and hyperoxemia might be a higher risk than hyperoxemia alone. There are trials of simulations to those cycling effects in different animal models. In the neonatal ICU there is no clear cut-off to delineate the “sweet-spot" in the oxygen saturation zone; how much is too much has not yet been clearly defined.
In mice, the episodic “fluctuation” of hypoxia and hyperoxia during the induction of BPD potentiated the oxidative stress in lung tissue and exacerbated the alveolar developmental arrest.19These results suggest that the aggressive prevention of hypoxemic episodes in human neonates at risk for the development of BPD needs to be further investigated. Our group, along with other groups, has studied the role of RAS in local tissue injury,59,92,125–130 and showed that ACE-2 might be beneficial to attenuate lung injury.3,65,73 A future study by our group is planned to look at the effect of cycling of hypoxemia/normoxemia/hyperoxemia and the effect of ACE-2 in a small animal model of BPD. Measurement of partial pressure of oxygen and/or SPO2 is planned to better assess and quantify the effects of hypoxemia with that of hyperoxemia/hypoxemia.
None.
This work was supported by the Department of Pediatrics and Human Development at Michigan State University through start-up funds awarded to Dr. Tarek Mohamed.
Authors declare that there is no conflict of interest.

©2020 Mohamed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.