Journal of
eISSN: 2376-0060


Brief Report Volume 8 Issue 4
1University of Health and Humanities,Virgin Islands, USA
2Department of Veterinary Medicine, Madingley road University of Cambridge, UK
3University of Science Arts and Technology, Monserrat, USA
Correspondence: R. Awan, Dean and Professor, University of Health and Humanities, Tortola. British Virgin Islands Life member Darwin College University of Cambridge UK
Received: December 22, 2021 | Published: December 31, 2021
Citation: Awan A, Tulp OL, Fields HJ. No protection seen on challenge with live virus after single intranasal immunization with heat inactivated virus in murine model of EHV-1 infection. J Lung Pulm Respir Res. 2021;8(4):163-168. DOI: 10.15406/jlprr.2021.08.00269
Equine herpes virus (EHV-1) causes respiratory disease, abortion, neonatal death, paresis, retinopathy, and latent infection and is wide-spread among equine worldwide. Horses show transient immunity after natural or experimental EHV-1 infection and immune responses to EHV-1 begin to decline after a few months after infection. As a result, recovered horses are prone to subsequent EHV-1 infection. Due to transient immune responses, effective and lasting vaccination remains a challenge. As this virus is widespread among equine, development of effective vaccine is a challenge. We used a murine model to study the efficacy of heat inactivated virus in terms of protection in a challenge study. After 34 days following intranasal inoculation with heat inactivated virus, mice were challenged with live virus along with previously placebo control group. Clinical signs, virus titres, and viraemia were studied in both groups. We noticed that mice on challenge showed more clinical signs at peak of infection but no significant difference in virus titres and infectious centre assay was noted. The results of this study suggest that heat inactivated virus does not provide any protection to challenge dose but in fact these group looked clinically worse. These results are discussed along with the possible mechanism involved in more clinical signs seen on challenge after single dose of intranasal immunization by heat inactivate virus in current communication.
EMEM, eagles minimum essential medium; RK-13, rabbit kidney cell line; EEL, embryonic equine lung cells; FCS, fetal calf serum; CPE, cytopathic effect; CD4, cell determinant 4 (lymphocyte cell expressing molecule 4); CD8, cell determinant 8 (lymphocyte cell expressing molecule 8); CD19, Cell determinant 19 (lymphocyte cell expressing molecule 19); CD20, Cell determinant 20 (lymphocyte cell expressing molecule 20); FITC, fluorescein isothiocyanate; CTL, cytotoxic lymphocyte; DPI, days post infection; M.O.I., multiplicity of infection; IF, immunofluorescent staining; IP, intraperitoneal; TEM, transmission electron microscopy
Equine herpes virus type 1 is transmitted in equine as respiratory infection throughout the world and is widespread among horses. This virus replicates in the respiratory epithelium lining including the turbinate bones, trachea bronchioles, ciliated and non-ciliated cells and pneumocytes type 1and lymphocytes type II, causes cell associated viraema. Viraemic cells can spread the virus throughout the body and can crosse the placenta to the foetus leading to abortion and to the CNS leading to myeloencephalopathy and paresis.1-10 Equine herpes virus is an alpha herpesvirus and like other alpha herpes viruses becomes latent, and virus could be reactivated at a later time. Virus reactivation during period of stress causing clinical disease and virus shedding has been reported in horses or after administration of corticosteroids.3,6,11 Natural immunity to this virus after natural infection in horses or experimental infection in SPF foals is short lived and horses could be re-infected with the same strain of virus. Thus, it is a great challenge to control the spread of this infection in horses due to a transient and short-lived immunity. This virus is so widespread and ubiquitous among horses that it is so difficult to obtain a horse free of EHV-1 infection to study the pathogenesis. A murine model was established to study pathogenesis and immune response to this infection.12,13 This model since then has been widely used to study pathogeneses, latency vaccine efficacy and antiviral efficacy.14-18
Most of the information about the pathogenesis of herpes simplex virus (HSV-1 and 2) came from a murine model of HSV infection. One prominent observation noted in mice after HSV infection is that mice become refractory to second HSV infection as immunity is solid and long lived 19.19-21 Similar responses may be seen in human subjects as subjects who recovered form infection cannot be easily re-infected by similar strains but show symptoms of the disease after reactivation. In many cases if one partner is showing reactivation other partner may not contract the illness by intimate contact due to the presence of immunity (personal observation of married medical students).
In herpesvirus infection the nature of humoral immune response is very complex, and many elements of antibody and cell-mediated immune responses are involved in combating the viral infection. Humoral antiviral immune is important not only in neutralizing extracellular virus but also in cooperating with non-specific effector cells such as natural killer cells and macrophages to lyse the infected cells.22 These cells also play part in antibody dependent cellular cytotoxicity. Humoral antibodies are relatively ineffective against intracellular virus spread which is characteristic of herpesvirus infection, and which virus can infect neighbouring cells without being in extracellular spaces and cell-mediated immunity responses have been shown to be effective in limiting these infectious.21-28
In the mouse model of EHV-1 infection, like in the natural host, we noticed a transient humoral immune response. Humoral immune responses started to decline after 4 weeks of infection but retained a vigorous cellular response as measured by DTH (Awan et al. 1990). Although immunity to herpes virus infection is generally more dependent upon the cell mediated immune responses than humoral responses so far, the resistance to reinfection in EHV-1 has only been correlated with the humoral antibody response. As mentioned above the humoral immune responses to EHV-1 infection is relatively short-lived and reinfection can take place within 3-5 months of previous exposure. An affective immune response requires a synchronization of several different physiological and immunological events. Vaccination, a form of prophylaxis has been phenomenally successful in control of other viral infection either as an inactivated virus such as rabies or Polio, or a subunit vaccine (hepatitis B). In view of the impressive record of vaccination programs worldwide it seems surprising that attempts to make an effective vaccine against EHV-1 have been disappointing and challenging. Means to protect horses from re-infection depend on the antigenic stimulation of antiviral immune response of the horse either with live or inactive vaccine both of which have been tried.1,2,29
Although infection is followed by the appearance of neutralizing and complement fixing antibodies it appears that the mere presence of these antibodies is not sufficient to guarantee protection and thus re-infection could occur in the presence of such antibodies. How the virus escapes the presence of such antibodies is not yet known. it seems surprising but understandable that attempts to make an effective vaccine against EHV-1 have been very disappointing.30-33
In this study mice were given intranasal heat inactivated virus and challenged with live virus and protection was studied in terms of virus titres, histopathology of lungs and presence of viraemia. Furthermore, mice who were challenged showed severe clinical signs and a possible mechanism of these clinical signs in the challenge study is discussed in this communication.
Mice strain
Female BALB/C mice were obtained from Bantin & Kingman, UK. Mice were 3-4 weeks old on arrival were maintained for one week in pre-sterilized plastic cages with pine fine shavings as bedding in a conventional 16/8 light cycle at room temperature [20⁰C] to acclimatize to the new surroundings and to minimize the effects of transportation and environment before any regulated experimental procedures were performed.
Virus strain and tissue culture
EHV-1 strain AB4 was a gift from Professor Neil Edington of the Royal Veterinary College, London, UK. This strain of EHV-1 was originally isolated from a case of equine herpes with neurological complication (paresis). The virus was grown in rabbit kidney fibroblast (RK-13). The RK-13 monolayers were grown in Eagles’ minimum essential medium (EMEM) supplemented with 10% newborn fetal calf serum (FCS). Cell culture was grown at 37⁰C in a humidified atmosphere containing 5% CO2. Virus was propagated in RK-13 cell in EMEM supplemented with 2% FCS at a low multiplicity of infection (m.o.i.) and the working stock was stored at -70°C in small volumes until used.
Intranasal inoculation of mice
BALB/c female mice were inoculated (107 p.f.u per mouse) intranasally and observed daily. Mice were slightly anaesthetized with ether and 20 µl in volume of virus suspension was placed in each nostril until all was inspired, which occurred within few seconds. When all mice had been inoculated, the surplus virus was titrated to confirm the dose administered.
Infection of mice after primary intranasal inoculation with heath-inactivated
Mice were divided in three groups. Group 1 was inoculated with live virus 107p.f.u. / mouse, Group II was inoculated with heat -inactivated virus 107 p.f.u. erstwhile per mouse and group III was inoculated with an uninfected RK-13 cell lysate. Approximately 70 percent of the mice died in groups 1 and remaining mice in this group were used to study virus pathogenicity. No mortality was seen in group II or group III.
Groups II and III were used in the challenge study. Thirty-four days after the initial inoculation, all mice in groups II (heat inactivated group) and group III (RK cell lysate placebo group) were inoculated with same dose of virus (107 .4 pf.u./ mouse). Clinical signs were noted and compared. Five mice were humanly euthanized on days 3 and four mice each on days 5 and 8 post infection from both groups. Their respiratory organs were removed and processed for virus isolation. Anticoagulated blood (EDTA) was also collected and infectious centre assays for viraemia was performed to compare viraemia (see below).
Clinical assessment
Mice were examined daily and weigh individually. Their clinical signs were noted subjectively. Obvious signs such as ruffled fur, crouching in corners, dyspnea, abdominal breathing, hunch back, dragging movements and deaths were recorded.
Virus isolation from murine tissues
Group of mice from each infected group were sacrificed by pentobarbitone sodium injection. The various respiratory organs were minced with scissors and homogenized in an electric blender in 2 ml quantity of EMEM. The suspension was sonicated for 1 min at 0oC and centrifuged at 3000 rpm for 10 min at 0 oC to remove cellular debris. Dilutions of the supernatants was performed in EMEM, and sample were inoculated on to confluent RK-13 monolayers. After 45 min of adsorption, EMEM containing 2% foetal calf serum (FCS) and 1% carboxymethyl cellulose (CMC) was added, the monolayer cultures were incubated and examined after 2 to 3 days. Monolayers were stained with crystal violet and plaques were counted.
Infectious center assay
Blood (2mg/ml EDTA) was collected direct from the heart following the induction of terminal anesthesia. The blood was centrifuged in microfuge tubes for 5 min and the buffy coat was remove by micropipette. The buffy coat was mixed in 0.9 ml of sterilized distilled water for 1 min to lyse the erythrocytes (flash lysis). The osmotic balance was restored with sterilized PBS X 10 at 1/10 volume of buffy coat suspension in “whirlimix” and centrifuged for 5 min to pellet the buffy coat cells. The cells were counted in a haemocytometer and a precise number of leucocytes were then added to the confluent RK-13 monolayer. After 30 min of incubation to allow the cells to settle down onto the monolayers, the medium was overlay with 5% FCS added and incubation continued for a further 5 days. The development of plaques was determined and number of plaques representing infectious centres per 106 cells were counted. In the absence of cytopathic effect or plaques, monolayers were harvested and centrifuged at 3000 rpm for 10 min to pellet the cells. These cell pellets were mixed in virus isolation medium and sonicated in ice cold water in sonic bath for one minute, pipetted onto fresh monolayers and incubated for development of any plaques. One plaque represents one infectious centers.
Postmortem findings and Histopathology studies
Gross appearance of lungs including any consolidation, colour changes or spongy feel of lung was checked and recorded. Small sectioned of lung were fixed in 10% formal saline, embedded in paraffin, and thin (5µ) sections were cut and stained with H and E staining to evaluate histopathological changes in both the groups.
Clinical signs after intranasal inoculation
All infected mice started to show clinical signs of infection 48 hours after inoculation and by day 3 all were hunched with ruffled fur and infected animal apparently looked smaller and started losing weight which was confirmed by measuring the weights of each mouse. Infected mice showed continuous weight reduction for 4 to 5 days and then gradually started to recover. From third day irregularity in breathing was noted which got worse by day 5 post infection. About half of the animal recovered from infection. Mice were also placebo infected with cell lysates only. None of this group showed any loss in weight or any other signs of reaction to monolayer cell lysate.
After virus challenge the groups of mice previously inoculated with heated-inactivated virus showed more clinical signs and difficulty in breathing (dyspnea, abdominal breathing) compared to mice who had been exposed to the virus for the first time.
Postmortem finding
The lungs of mice who has seen the virus for the first time showed typical occasional consolidation but mice who had seen the virus second time showed more severe signs of consolidation and in some cases the lung was so badly consolidated, it lost the spongy feel of a lung and showed a more solid texture.
Virus isolation from respiratory tissues and infectious centers assays (viraemia)
Virus was isolated from respiratory tissues (turbinate bones and lungs) from both the groups. Though lower virus titers were obtained from the group of mice that were challenged with live virus. After primary heat inactivated virus was inoculated to group of mice who were inoculated with the virus for first time showed no significant difference (p>0.05) in virus titers were found in the turbinate bones or lungs between both the groups (Figure 1, Table 1). Similarly infectious centers (viraemia) were found to be very similar (Table 2). This contrasted with the clinical signs noticed in challenge groups as described above. Similarly, there was significant reduction in the weight of mice in this group compared to groups who were given the virus for the first time.
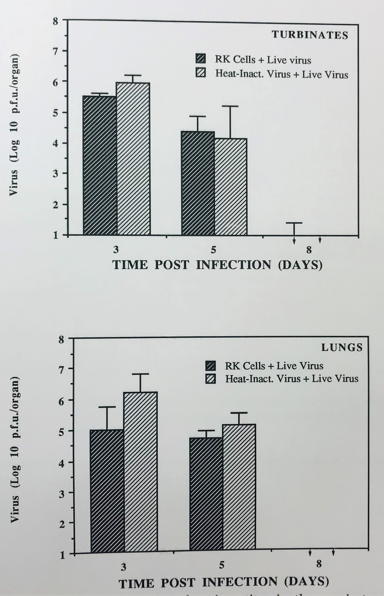
Figure 1 Histogram showing the virus titre in the respiratory tissues of mice previously inoculated with heat-inactivated virus (107 erstwhile p.f.u. /mouse) or RK-13 cells Lysate. Both the groups were challenge with live virus 10 7.4 p.f. u/mouse 34 days later and their organs were tested on days 3, 5, an 8-post infection. Each bar represents the virus titre (geometric means + S.D) of five or four mice processed at each time point.
|
Virus titres (log 10 p.f.u./organ±S.D.) |
||||
|
Days P.I. |
RK+Live Virus |
Live Virus+Live Virus |
||
|
|
Turbinates |
Lungs |
Turbinates |
Lungs |
|
3 |
4.2 ± 0.64 |
6.3 ± 0.13 |
4.0 ± 0.55 |
5.0 ± 0.07 |
|
5 |
3.0 ± 0.69 |
2.1 ± 1.67 |
1.45 ± 0.25 |
<1 ± 0.0 |
Table 1 Table showing the virus titres in the turbinate bones and lungs. Mice were previously mock infected with heat inactivated virus (10 7.0 erstwhile p.f.u./mouse) or with RJ -cell lysate and were superinfected with 10 7.4 p.f. u. mouse after 34 days post inoculation. These data represent the groups of five mice processed individually on each time point
|
Individual |
Time post infection days |
||
|
|
3 |
5 |
8 |
|
1a |
0b |
38 |
0 |
|
2 |
23 |
16 |
0 |
|
3 |
0 |
24 |
0 |
|
4 |
3 |
2 |
0 |
|
5 |
114 |
N.D. |
N.D. |
|
6 |
4 |
28 |
0 |
|
7 |
0 |
32 |
0 |
|
8 |
10 |
26 |
0 |
|
9 |
16 |
8 |
N.D. |
|
10 |
24 |
N.D. |
N.D. |
Table 2 Table showing the infectious centre forming cells in the blood of mice challenged with live virus previously mock -infected with heat-inactivated virus. Mice were inoculated intranasally with EHV-1 107 erstwhile p/f/u/mouse heat inactivated (mice 1-5) or mock infected with RK13 lysate (mice 6-10). Thirty-four days after the primary infection, both groups were infected with 10 7.4 p.f. u/ per mouse. This data represents the results obtained for individual mouse infectious centres from leucocytes (number of infectious centre forming cells /10 6 leucocytes
Histopathology
The histopathological observations in made in the both groups showed the type of cytopathology of EHV-1 infection, e.g. desquamation of epithelial cells lining the bronchioles and loss of normal histological alveolar architecture as described previously. However, subjectively was a greater degree of peribronchiolar / perivascular cuffing and local infiltration of inflammatory cells in the groups of mice previously inoculated with heat - inactivated virus.
Equine herpes virus (EHV-1) causes respiratory disease. The virus replicates in the respiratory tissues from lining to the turbinate bones, ciliated and non-ciliated cells lining the trachea bronchi bronchioles and pneumocytes type I and type II, causes viraemia and abortion, neonatal death, myeloencephalitis, paresis retinopathy.1,3,6,34,35 This virus is an alpha Herpesviridae and like other alpha herpes viruses becomes latent and reactivates and is widespread among equine worldwide.36-38 One of the biggest challenges is that horses show only a transient immunity after natural or experimental EHV-1 infection and humoral immune response and after infection the humoral immune responses begin to wane and the horses can become reinfected.1,17,39-40
A murine model for equine herpes virus type 1 was established, and a similar pattern of virus replication was seen in the murine model i.e., virus replication in the respiratory tissues, viraemia. latency, a transient humoral immune response, and abortion.12-18,32 Furthermore, humoral immune responses started to decline after one month of acute infection, but cellular immune responses were still active after 78 days of acute infection, the latest time point tested in this study.12 Mice could be reinfected with EHV-01 after they recovered (Awan et al unpublished observation).
Most of the information regarding many infectious diseases are obtained from animal models. Murine model of human herpes virus infection provides a valuable information regarding pathogenesis, immune responses, latency, vaccine efficacy and antiviral efficacy.21,26,27,41-43
Mice cannot be re-infected once they recovered from HSV-1 infection. Virus also becomes latent in the trigeminal ganglia, can reactivate and cause disease. Studies in mice predict the similar patten in human.41,44 After primary infection of herpes simplex virus infection there is a strong immune response and human subjects are not easy to be re-infected with the virus. Similarly, latently infected subjects are protected from a second infection or reinfection from HSV, but virus reactivate and cause disease.21,26,27 This is closely observed in married subjects where one partner reactivates and shows the signs of the disease, but other partner may never become reinfected or show any signs of disease perhaps due to primary subclinical infection and/or presence of immunity. We know that about 88 percent or more human subjects in the western world could get infected with herpes simplex type 1 and the virus becomes latent (detection of Latency associated transcripts in trigeminal ganglia of cadavers), but all do not show reactivation from latency. Reactivation from latency is a complex mechanism and depends on combination of many stress factors, lifestyle, place of living (people living in sunny and high mountains show more reactivation due to UV light exposure) and immune response.20,28
In equine herpes virus infection in horses, although the infection is followed by the appearance of neutralizing and complement fixing antibodies, it appears that the presence of antibody is not sufficient to guarantee protection and reinfection can occur in the presence of such antibodies.33 Furthermore, humoral responses started to decline and due to transient antibody response, horses could be reinfected after 3-5 months of previous infection. Such mechanism of decline in antibody response is still not understood, but possible mechanism is discussion in a different communication (Awan et al. manuscript in preparation).
In the present study mice were inoculated with heat inactivated virus (which mimic vaccination) and challenged with live virus 34 days later. Though there was slightly less virus obtained from challenged mice, but the difference was not significant in virus titres in respiratory tissues in group of mice who were inoculated with virus for the first time to a group which was inoculated with heat inactivated virus and challenged with live virus (Figure 1, Table 1). Furthermore, the infectious centers in blood (state of viraemia) were found to be similar in both groups (Table 2). However more clinical signs (by subjective evaluation) were seen in the group of mice which was inoculated with heat inactivated virus and challenged with live virus compared to group who was inoculated with the live virus for the first time. Furthermore, challenged mice also lost more weight. On histopathological examination of lung tissues, more histopathological changes were observation in mice previously inoculated with heat inactivated virus and challenged with live virus (subjective evaluation). We wished to investigate this further and role of inflammatory cells on challenge so in order to address this, further experiment was conducted where mice were given live virus and challenge with live virus after five months and this is subject of future communication (Awan et al manuscript in preparation).
We have seen a strong cell-mediated response (delayed type hypersensitivity response or DTH) after 78 days of infection in murine model of EHV-1 and this could be further strengthened by local infiltration of the inflammatory cells and peribronchiolar cuffing in the challenge groups lung tissue.12 We speculate that it is DTH, or cytotoxic cells are the one which are causing more damage to the lung parenchyma when virus started replicating in the cells and expressed MHC-1 peptides for CTLs. This has been reported in other studies.24,25,46-52
Many research groups who are working on vaccine for respiratory viruses or respiratory disease s (influenza, RSV, or asthma) did the challenge study after intranasal infection and showed protection in their own way. By looking at the data presented in conference all studies showed weight loss and even death in challenge study, but none could explain this loss of weight as none went any further to investigate the pathological changes seen in those mice who lost weight or died. We are fortunate that we went one step further and tried to find out the reasons of the clinical signs weight loss and death even though less virus was obtained after the challenge study. In order to address this question further experiment was performed in which instead of heat inactivated virus inoculation, mice were infected with live virus and challenged with live virus after five months of primary infection. We observed more clear answers as why there was more severe respiratory distress in mice challenged with live virus and this is a subject of future communication (Awan et al manuscript in preparation).
Contributors
This study was performed in department of veterinary medicine University of Cambridge, Dr. Hugh field is life fellow, Queens college and Dr. Aftab Awan is life member of Darwin College and Dean of College of Veterinary Medicine, University of Health and Humanities Tortola. Virgin Islands. Dr Tulp is a professor of Medicine and president of University of Science Arts and Technology and University of Health and Humanities Virgin Islands.

©2021 Awan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.