Journal of
eISSN: 2376-0060


Case Series Volume 8 Issue 1
1Department of Medical Oncology, Hassan II University Hospital, Morocco
2Department of Pathology, Hassan II University Hospital, Morocco
Correspondence: Lamyae Nouiakh, Department of Medical Oncology, Hassan II University Hospital, Fes, Morocco, Tel
Received: January 08, 2021 | Published: February 8, 2021
Citation: Nouiakh L, Benbrahim Z, Oualla K, et al. Malignant pleural mesothelioma: Experience of the Medical Oncology Department of Hassan II Hospital in Fez, Morocco. J Lung Pulm Respir Res. 2021;8(1):6-11. DOI: 10.15406/jlprr.2021.08.00242
Background: Malignant pleural mesothelioma (MPM) is a primary, rare and aggressive malignant tumor, closely correlated with asbestos exposure.
Aim: The objective of this work is to study the main epidemiological, clinical, histological and therapeutic aspects of these tumors and to compare our results with those reported in the literature.
Methods: We conducted a retrospective study of 8 cases of MPM collected at the medical oncology department of CHU Hassan II in Fez, for a period of 8 years from January 2011 to January 2019.
Results: The mean age at diagnosis was 53 years with an F/H sex ratio of 1.7. Occupational exposure to asbestos was found in only one patient. Pleural effusion syndrome was present in all patients. Biopsy was done under thoracoscopy in 7patients (87.5%). MPM were mostly stage IV (50%). The standard treatment was conventional chemotherapy based on platinum, administered to all patients. After an average number of 4 courses, the objective response rate was 12.5%, and the disease control rate was 50%. Second line chemotherapy consisted of monotherapy with NAVELBINE (67%) or PEMETREXED (33%). After a median follow–up of 17 months, the median of progression–free survival was 5 months, and that of overall survival was 8 months.
Conclusion: Based on the results of this study, we conclude that MPM is a very rare tumor in Morocco with poor prognosis. Prognostic factors affecting survival could not be studied due to the low number of patients included in the study, and the lack of data on medical records.
Keywords: pleura, mesothelioma, asbestos
Malignant pleural mesothelioma (MPM) is a rare primary tumor, developed from the mesothelial cells of the visceral or parietal pleura. The most frequent location is at the pleura (90% of case). The other locations being rarer: peritoneum (5 to 10% of cases), pericardium (0.4% of cases), and very rarely the tunica vaginalis testes. The incidence of this type of tumor varies from country to country in the world; in the United States,1 the incidence is estimated at 3,200 cases / year, while in France, MPM affects around 800 to 1,000 people each year with an incidence of around 16 cases / million inhabitants / year.2,3 It is a pathology that is mainly due to exposure to asbestos, whether or not associated with other etiopathogenicfactors.4–6 MPM is characterized by a long latency period that can go up to 20 to 40 years.3,7 It has a very poor prognosis with a 5–year relative survival rate of 7%,8 and a median survival of 8 to 14 months.9,10 Late diagnosis, the lack of an effective therapeutic approach and resistance to conventional chemotherapy make this prognosis poor.
The objective of this work is to study the main epidemiological, clinical, histological and therapeutic aspects of these tumors and to compare our results with those reported in the literature.
In order to describe the epidemiological, clinical, histological, and therapeutic profile of patients with MPM, we conducted a descriptive retrospective study on 8 cases of MPM collected at the medical oncology department of the Hassan II University Hospital in Fez, over a period of 8 years from January 2011 to January 2019. We only included patients treated in our department, aged over 18 years with a diagnosis of MPM made after an histopathological study. Statistical analysis was done by SPSS version 23software, qualitative variables are expressed in frequency and percentage, and quantitative variables are expressed as median and mean.
Between January 2011 and January 2019, we included 8 patients with MPM in the medical oncology department of CHU Hassan II in Fez. It represents less than 0.1% of all cancers diagnosed during the study period. The mean age at diagnosis was 53 years with age ranges ranging from 26 to 80 years. There was a slight male predominance with a sex ratio M/F of 1.7.
Occupational exposure to asbestos was found in a single patient, smoking was noted in 3 patients (37.5%). The concept of irradiation was not reported nor family cases of pleural mesothelioma.
Clinical data
All of our patients presented respiratory symptoms dominated by dyspnea (6 patients; 75%), chest pain (5 patients; 62.5%), and cough (2 patients; 25%). Pleural effusion syndrome was present in all patients. Five patients (62.5%) were in good general condition performance status (PS 0–1) at the time of diagnosis, while three patients had advanced pleural mesothelioma (37.5%) had a PS of 2.
Radio–histological data
Thoraco–abdominal–pelvic tomodensitometry was performed in all patients. According to the TNM classification, MPMs cases were mostly stage IV (50%), 3 patients (37.5%) were classified as stage III, and only one patient was classified as stage IIb. The elective sites of metastasis were: lymph node (50%), lung (37.5%), and pericardium (12.5%). FDG–PET–CT, and new generation spiral CT were not performed in our patients.
The diagnosis of MPM was confirmed by pleural puncture biopsy in a single patient, and in 7 patients (87.5%), the biopsy was performed under thoracoscopy. The pathological study was in favor of malignant pleural mesothelioma with an undetermined histological subtype in 7 patients (Figures 1–3), the epithelioid form was found in a single patient. Pleural fluid cytology was not been done.
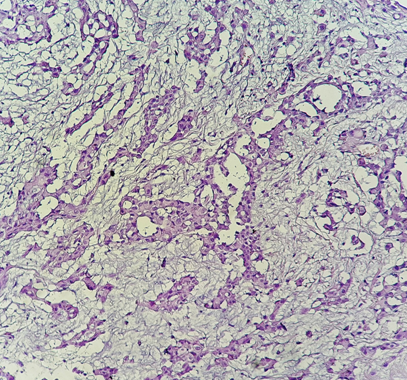
Figure 1 Histological study: HES*200: Carcinomatous proliferation arranged in spans and tubes (pathology department of CHU Hassan II in Fez).
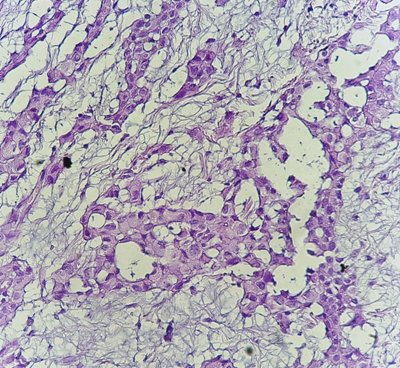
Figure 2 Histological study: HES*400: the tumor cells are large in size with large nuclei. (Pathology department of CHU Hassan II of Fez).
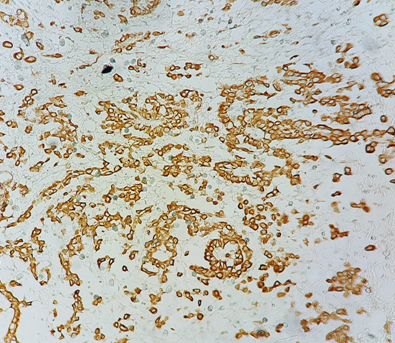
Figure 3 Immunohistochemical study: Diffuse labeling of tumor cells by anti-calretinin antibody (pathology department of CHU Hassan II in Fez).a
Therapeutic data
Radical surgical treatment could not be performed due to the unresectability of the tumor in patients with localized tumor. 5 patients received local palliative thoracoscopic treatment: pleural talcage was performed in half of our population (50%), and only one patient underwent decortication of the pleura. Two patients (25%) had an indication for palliative radiotherapy. The standard treatment was based on conventional chemotherapy, administered to all patients. All the patients received a regimen based on platinum salts (cisplatin 75mg/m2/3W) as first–line combined with either PEMETREXED (500mg/m2/3W) (5 patients; 62.5%), or GEMCITABINE (1250mg/m2 d1, d8, d1=d21) (2 patients; 25%) or DOXORUBICIN (60mg/m2/3W) (1 patient; 12.5%). After an average number of 4cures, the objective response rate was 12.5%, and the disease control rate was 50%. Three patients (37.5%) were able to receive 2nd line chemotherapy, this chemotherapy consisted of monotherapy with NAVELBINE (67%) or PEMETREXED (33%). The side effects induced by chemotherapy were mainly haematological (febrile neutropenia (37.5%), anemia (25%), and gastrointestinal (25%) complications. Analgesic treatment was administered in all patients. After a median follow–up of 17 months, the median progression–free survival (PFS) was 5 months and that of overall survival (OS) was 8 months.
Table 1 summarizes the characteristics of the patients in our study.
Characteristics |
|
Number(%) |
Gender |
Male Female |
5(62.5) 3(37.5) |
Age |
<40years > 40years |
2(25) 6(75) |
Risk factors |
Smoking |
3(37.5) 0(0) |
Respiratory clinical signs |
Dyspnea |
6(75) |
Performance status (PS) |
0-1 |
5(62.5) |
Diagnostic methods |
Thoracoscopic biopsy |
7(87.5) |
Prognostic classification |
IIb |
1(12.5) |
Metastatic sites |
Nodes |
4(50) |
Palliative treatment |
Talcage |
4(50) |
Conventional chemotherapy |
Platinum salts + PEMETREXED |
5(62.5) |
Table 1 Characteristics of patients in our series
MPM is a relatively rare disease; its incidence is estimated at 2 cases/million inhabitants/year with variations depending on the country of the world.11 This incidence is gradually increasing, this increase is due to the continued use of asbestos fibers, especially in underdeveloped countries. 2,12,13 In our work, MPM represents less than 0.1% of all cancers diagnosed during the study period, which testifies to the rarity of these tumors, also reported in the literature.
According to some authors, MPM has been shown to preferentially affect men, due to exposure to asbestos in the workplace where the majority of workers are male.14,15 Other authors have shown through epidemiological studies4 that the incidence of MPM is higher in women than in men even in the absence of direct occupational exposure to asbestos.16 This was explained by a para–occupational exposure to asbestos, the latter could be found in buildings, the ground, the clothes of asbestos workers, which can be at the origin of the appearance of MPM in combination with other cofactors.17 In our study, the majority (62.5%) of affected patients were men.
One of the particularities of MPM is the long latency period between exposure to asbestos and the onset of the disease, which explains why the majority of affected patients are over 60 years old with peaks between 80 and 84 years for men and between 75 and 79 years for women.18,19 MPM can also affect children and young adults, but there are no data on the incidence of this disease in this population. Based on data from an autopsy series, the rate of pediatric mesothelioma was estimated at 2 to 5% of all mesothelioma cases and represents 0.5 to 1 case/ 10 million/ year.20 In the literature, few cases of pediatric MPMs and in young adults have been published; there are a few reports and old articles.21–26 In our study, the average age at diagnosis was 53 years.
Exposure to asbestos fibers is the essential etiopathogenic factor in the development of MPM. The link between asbestos exposure and the development of the disease was clearly identified and proven in the early 1960s.19 Exposure to ionizing radiation could be responsible for the development of MPM.6 Radiation exposure for the treatment of lymphoma, prostate cancer, and Wilms tumors has been shown through studies to be responsible for the development of MPM2.27–29 Another environmental factor has been implicated in the development of MPM which is Simian virus 40, studies have shown an association between MPM and oncogenic simian virus 40 (SV40).30,31 The anti–SV40 antibody has been found in 60% of patients with mesothelioma,32 these data have reinforced the link between MPM and SV40. Genetic factors in particular the loss of expression of BAP1, or of protein associated with BRCA1 were involved in the genesis of MPM, the presence of mutations in these genes favors the occurrence of pleural mesotheliomas coexisting with exposure to asbestos.33 Genetic predisposition seems to play a role in the development of MPM due to the existence of familial cases of "mesothelioma”.19 This factor could alone or in association with asbestos exposure be the genesis of the disease.34–36
The role of tobacco intoxication in the development of MPM has not been found in the literature. In terms of the etiopathogenic factors that were found in our study: the notion of exposure to asbestos was reported in a single patient and there was no history of irradiation or family cases of mesothelioma, this could be explained by the low number of patients included in the study.
The clinical signs found in our study are generally the same as those reported in the literature; these signs are not very specific and present late during the course of the neoplasia. The symptoms most frequently found in the literature are: dyspnea and chest pain (90%) related to the presence of pleural effusion, other signs are inconstant such as asthenia (36%), cough (22%), sweating and chills (22%).37
Regarding the imaging data, computed tomography (CT) scan is superior to the chest X–ray, it remains the key presumptive diagnostic examination of the MPM. It allows visualization of tumor pleural thickenings which are irregular, often circumferential and enhanced by the contrast with associated fluid reaction. It makes it possible to study the diaphragmatic, pericardial, and mediastinal pleura, and the condition of the fissures. It also helps to assess any damage to the chest wall, mediastinal structures, such as nodal and ganglia metastasis.38 MPM is characterized by a local evolution with the lesions extending gradually towards neighboring structures; in some cases it may have an initial lymph node presentation.39 Metastatic spread is most often lymphatic, rarely hematogenous.38
In our study, chest computed tomography revealed pleural thickening in 7 patients and mediastinal lymphadenopathy in 4 patients.
The most efficient examination for a definite histological diagnosis of MPM with sensitivity of 90% is still video assisted thoracoscopy. It allows the pleural cavity, mediastinum and pericardium to be explored, thus allowing large, deep and numerous biopsies to be performed. In our series, it was performed in 7 patients. Trans–parietal biopsies under radiological or ultrasound identification can be performed but the diagnostic yield remains low at around 30%. They are only recommended if thoracoscopy is contraindicated. Pleural puncture makes it possible to perform a cytological study that can guide the diagnosis, but it remains insufficient to establish a definite diagnosis and to specify the histological subtype which constitutes one of the prognostic factors of MPM.38 In our study, it was carried out in a single patient because the other techniques could not be carried out given the altered general condition of the patient.
From a histopathological point of view, there are three histological variants of MPM:40 the epithelioid subtype is the most frequent form; it represents 50 to 70% of cases. Its prognosis is the least unfavorable among the three forms, it is a chemosensitive histological entity which explains the prolonged survival in patients with this type of tumor, and the median survival can be up to 11 months. The biphasic subtype is a variant that accounts for 25–30% of MPMs. Its prognosis is less good than the epithelioid subtype; the median survival can reach 7 months. The last subtype is that of the sarcomatoid, it accounts for 10–20% of MPM; it is the most aggressive form. Its prognosis is reserved with a median survival of 3 months. 41
The immunohistochemical study is essential for the diagnosis of MPM, especially for differential diagnosis. The 3 most used positive markers are CK 5/6, calretinin and WT1. In our study, the epithelioid form was found in a single patient. For the rest of the patients, the complete pathological study could not be performed due to the small size of the samples.
On the therapeutic level, the treatments that are currently offered; we cite surgery, radiotherapy and systemic treatment. The decision on the therapeutic choice must be taken collegiate in a multidisciplinary consultation meeting. Due to the diagnostic delay, systemic treatment remains the only therapeutic modality which appears reasonable and which could increase the survival of patients with MPM. Almost all chemotherapy regimens have been tested.42,43 diets containing platinum salts have been shown to be effective compared to those without. Based on data from the EMPHACIS study published in 2003 by Vogelzang and colleagues,44 the CISPLATINE (75mg/m2/3W)–PEMETREXED (500mg/m2/3W) combination has become the gold standard treatment in patients with locally advanced unresectable or metastatic MPM. As part of treatment with targeted therapies, and to improve the response rate and objective results under chemotherapy, receptor tyrosine kinase inhibitors have been studied in different trials, but the results of most of these studies were negative.45 The best results with targeted therapies were found with BEVACIZUMAB, the MAPS trial conducted by Zalcman and his colleagues46 evaluated the addition of an anti–angiogenic agent to standard treatment, the benefit in terms of survival was 2.7 months, statistically significant in favor of triple therapy. As this therapeutic regimen has shown superior efficacy with manageable toxicity, it is included among treatment alternatives, although it has not yet been validated as a first–line treatment.
For the place of immunotherapy in the treatment of MPM, immune checkpoint inhibitors such as anti–cytotoxic T lymphocyte–associated protein–4 (CTLA–4) monoclonal antibodies or monoclonal antibodies targeting programmed cell death–1 (PD–1) or its main ligand PD–L1 have been tested in MPM, either alone or in combination with chemotherapy.47 They did not show an impressive clinical response like in other solid malignant tumors. However, the first–line NIVOLUMAB–IPILIMUMAB combination proved to be more effective compared to chemotherapy in the CHECKMATE 743 trial. The trial demonstrated a statistically significant improvement in overall survival (OS) for patients treated with nivolumab plus ipilimumab compared with those who received chemotherapy. Median OS was 18.1 months (95% CI: 16.8, 21.5) versus 14.1 months (95% CI: 12.5, 16.2) (HR 0.74; 95% CI: 0.61, 0.89; p=0.002).
In our study, all our patients received a regimen based on platinum salts, the majority of patients (62.5%) of which received the standard regimen (CISPLATINE–PEMETREXED), while 37.5% received either GEMCITABINE or DOXORUBICIN at place of PEMETREXED by breaking the latter.
For patients with early stage MPM, curative surgical resection remains a subject of controversy, as it is almost impossible to perform cancer surgery. The 2 surgical methods that are proposed are pleurectomy / decortication and enlarged pleuropneumonectomy (EPP), the choice of such a surgical procedure depends on several factors, in particular, the general condition and the choice of the patient as well as the expertise of the patient operator. The authors of the study conducted by Flores48 did not demonstrate the superiority of one technique over another, but morbidity and mortality was higher with PEP. In our study, all of our patients had diffuse MPM ineligible for radical surgery. Only one patient had a decortication of the pleura without pleurectomy.
The management of a metastatic pleural effusion begins with a diagnostic and evacuating pleural puncture, if necessary depending on its abundance. Performing iterative evacuating punctures then has a major impact on the quality of life of the patients concerned, linked to repeated hospitalizations, the constraints linked to the gesture and the resulting psychic repercussions. There are several approach techniques with each of the advantages, disadvantages. Among these techniques, we cite pleurodesis.
The purpose of pleurodesis is to produce multiple and strong adhesions in the pleural cavity between the parietal and visceral pleura in order to prevent recurrence of a pleural effusion using a chemical. Pleural surfaces should be irritated by the use of a sclerosing agent. The attack on the layer of the mesothelium is necessary for obtaining a good symphysis by triggering inflammatory reactions, recruitment and proliferation of fibroblasts and constitution of a fibrin layer between the two pleural layers. The most widely used products are talc, tetracycline and bleomycin.
Radiation therapy can be used as a treatment for MPM either as a neoadjuvant before PEP at a dose of 5–6 Gy49 or as an adjuvant at a dose of 50–60Gy.50 But, it is used mainly in a palliative situation.51,52 In our series, palliative radiotherapy was performed in 2 patients.
Supportive care must be systematically undertaken from the outset, with periodic reassessment, in particular for analgesic treatment.
Based on the results of this study, we conclude that MPM is a very rare tumor with a poor prognosis with an almost always fatal evolution. It was not possible to study the prognostic factors that condition survival due to the small number of patients included in the study, and the lack of data on patient medical records. To improve the management of patients with MPM, it would be interesting to carry out a large–scale study to prove in a statistically significant way the factors that influence survival, and to be able to carry out clinical trials with homogeneous groups of patients in order to propose patients receive adequate treatment which may improve the prognosis.
None.
None.

©2021 Nouiakh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.