Journal of
eISSN: 2376-0060


Research Article Volume 6 Issue 3
1Department of Pediatrics and Human Development, Michigan State University, USA
2Department of Physiology, Michigan State University, USA
Correspondence: Bruce D. Uhal, Department of Physiology, Michigan State University, 567 Wilson Rd, East Lansing, MI 48823, USA
Received: July 14, 2019 | Published: July 18, 2019
Citation: Abdul-Hafez A, Mohamed T, Uhal BD. Activation of mas restores hyperoxia-induced loss of lung epithelial barrier function through inhibition of apoptosis. J Lung Pulm Respir Res. 2019;6(3):58-62. DOI: 10.15406/jlprr.2019.06.00208
Background: Neonatal therapy with a high concentration of oxygen (hyperoxia) is a known cause of bronchopulmonary dysplasia (BPD). BPD is characterized by increased pulmonary permeability and diffuse infiltration of various inflammatory cells. Disruption of the epithelial barrier may lead to altered pulmonary permeability and airways fluid accumulation. Mas receptor is a component of the renin angiotensin system and is the receptor for the protective endogenous peptide angiotensin 1-7. The activation of the Mas receptor was previously shown to have protective pulmonary responses. However, the effect of Mas receptor activation on epithelial barrier integrity has not been tested.
Objective: To determine the effects of hyperoxia with or without Mas receptor activation on epithelial cell barrier integrity.
Design/Methods: Human epithelial cell line A549 was cultured on transwell polycarbonate porous membrane to confluence and treated with 95% oxygen (hyperoxia) for 72 hours with or without the Mas receptor agonist (AVE0991), or the apoptotic inhibitors Z-VAD-FMK or aurintricarboxylic acid. The cells were then challenged with Rhodamine labeled bovine serum albumin (Rh-BSA) on one side of the membrane. Fluorescent quantitation of Rh-BSA (albumin flux) was performed on the media in the other side of the membrane 3 hours later and was compared with 21% oxygen (Normoxia) control group. A549 cells were also cultured with or without AVE0991 in hyperoxia or normoxia and used for nuclear fragmentation apoptosis assay using propidium iodide staining.
Results: Hyperoxia induced an increase in albumin flux that was significantly prevented by AVE0991 treatment and by the apoptosis inhibitors. AVE0991 also significantly decreased the hyperoxia-induced nuclear fragmentation.
Conclusion: These results suggest that hyperoxia causes a disruption in the epithelial barrier integrity, and that this disruption is inhibited by the Mas receptor agonist AVE0991 through inhibition of epithelial apoptosis. These results reveal a novel potential drug for BPD and pulmonary edema treatment.
Keywords: bronchopulmonary dysplasia, pulmonary edema, hyperoxia, alveolar epithelia, angiotensin
Bronchopulmonary dysplasia (BPD) is a chronic lung condition that affects newborn babies who received high levels of oxygen for a long period or put on a ventilator after birth, especially those who were born prematurely. Infants develop BPD in about 1.5% of all newborn births.1 BPD is associated with neurodevelopmental deficits, cognitive impairments, failure to thrive, pulmonary hypertension and cor-pulmonale.2 High rates of in utero and perinatal exposure to infection may be causally related to preterm delivery and subsequent lung injury.3 Over the past two decades, the histological presentation of BPD has changed from heterogeneous pulmonary inflammation and fibrosis (Old BPD) to uniform arrest of alveolar development and variable interstitial cellularity and/or fibroproliferation (New BPD).4,5 In the new definition of BPD; lung development arrests before alveolarization, and the lungs have larger but much fewer alveoli than normal lungs. BPD is characterized by lung inflammation, airway injury secondary to interstitial and alveolar fluid overload, smooth-muscle hypertrophy, and oxidative stress.6 Inflammation and alveolar fluid overload is attributed to increased pulmonary permeability and diffuse infiltration of various inflammatory cells. Disruption of the epithelial barrier may lead to altered pulmonary permeability and airways fluid accumulation. The maintenance of barrier properties requires intact epithelial tight junctions.7,8 The apoptosis of alveolar epithelial cells (AECs) in vivo was shown to cause the collapse of the pulmonary epithelial barrier function.9-11
The local renin angiotensin system (RAS) is activated after tissue injury in a variety of organs to promote repair, but abnormalities in the process promote fibrosis.12,13 Previous studies have shown that the octapeptide angiotensin II (AngII) causes alveolar epithelial injury and apoptosis through its action on AT1 receptor.14-17 The recently discovered angiotensin 1-7 (Ang1-7) heptapeptide has shown a protective effect to pulmonary cells via its action on “Mas” receptor.18,19 The non-peptide compound AVE0991 is a Mas receptor activator that simulates the actions of Ang1-7 peptide,20 and has shown promise to attenuate lung injury.21,22
In the study presented here, we investigate the effects of hyperoxia on the epithelial barrier integrity and apoptosis. We also evaluate the protective effects of AVE0991 on the epithelial barrier.
Reagents and materials
Fluorescently labeled rhodamine (tetramethylrhodamine) bovine serum albumin conjugate (Rh-BSA) was purchased from Molecular Probes (Invitrogen). For the apoptosis inhibitors, the caspase inhibitor Z-VAD-FMK (ZVAD) was obtained from R&D systems, and the endonuclease inhibitor aurintricarboxylic acid (ATA) was obtained from Sigma-Aldrich. Corning Costar Transwell permeable support, 6.5mm insert, 0.4μm polycarbonate membrane, 24 well plates were purchased from Sigma-Aldrich. The Mas receptor agonist AVE 0991 from MedChem Express.
Cell culture
The human lung adenocarcinoma cell line A549 was obtained from the American Type Cell Culture Collection (ATCC) and cultured in Ham’s F12 medium supplemented with 10% fetal bovine serum (FBS) (Gibco). All experiments were conducted in serum-free Ham’s F12 medium. For Rh-BSA flux experiments, A549 cells were plated on Transwell membranes to obtain an intact monolayer in the inner chamber membrane in the presence of complete medium. Both inner and outer chambers were washed, replaced with serum-free F12 medium and were cultured for 72 hours in either 21%O2, 5%CO2 (Normoxia) or in 95%O2, 5%CO2 (Hyperoxia) in the presence or absence of 10-7M AVE0991, 60μM ZVAD, or 10μM ATA. DMSO solvent was added in control groups for absence of AVE0991, ZVAD, or ATA. After the 72 hours, the cells on the Transwell membrane were used for the Rh-BSA flux measurement.
Albumin flux
Integrity of the cultured cells epithelial barrier was determined by albumin flux similar to described previously 11. After treating the cells with the above mentioned conditions, treatment media was removed and tetramethylrhodamine-bovine serum albumin (Rh-BSA, 1μg/μl) in serum free F12 media was added into the inner chambers and unlabeled albumin in serum free F12 media into the outer chambers. Cells were incubated in 21%O2, 5%CO2 incubator. Three hours later, 50μl aliquots were taken from outer chambers into 96 well half area black plate. Rhodamine fluorescence from outer chambers was determined on FL600 fluorescence microplate reader (BioTek Inc., Winooski, VT).
Detection of apoptosis
Cells were cultured on 24-well cell culture plates to sub-confluent density and were exposed to normoxia or hyperoxia in the presence or absence of the Mas activator AVE0991 for 72 hours then were fixed in 70% ethanol. Apoptotic cells were detected by nuclear fragmentation assay using propidium iodide (PI) as described earlier,23 after enzymatic digestion of ethanol-fixed cells with DNase-free RNase in PBS containing 5 μg/ml PI. During fixation with 70% ethanol, detached cells were retained by centrifugation of the 24-well culture plates. Cells with discrete nuclear fragments with condensed chromatin were counted as apoptotic using epifluorescence microscopy. Apoptotic cells were scored over a minimum of four separate microscopic fields from each of at least three culture vessels per treatment group.
Mas activation prevents hyperoxia-induced increases albumin flux through alveolar epithelial cell monolayers
Figure 1 shows a significant increase in albumin flux through A549 cell monolayers cultured on Transwell inserts that had been exposed to 95%O2 or normoxia (21% O2). The increase in albumin flux was significantly decreased in the presence of the Mas activator AVE0991.
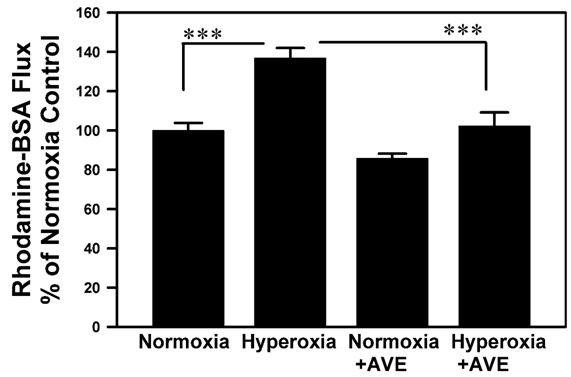
Figure 1 The Mas agonist AVE-0991 blocks Rhodamine-BSA flux across cultured alveolar epithelial cell monolayers caused by 95% oxygen. A549 cells were cultured on Transwell inserts to confluence as described in Methods and were then exposed to 95% O2 in the presence or absence of the mas activator AVE0991. Thereafter, Rh-BSA (1mg/ml) was added into the inner chambers and 50μl of aliquots were taken from outer chambers three hours later. Rhodamine fluorescence was measured and was normalized as percent of the control; see Methods for details. Bars are the mean +/- S.E.M.; *** p<0.001 using ANOVA Student-Newman-Keuls test, n=12.
Hyperoxia causes significant cell loss that is rescued by mas activation
Figure 2 shows that 72 hours exposure to hyperoxia reduces the cell number in A549 cell monolayers cultured on Transwell polycarbonate inserts, Moreover, AVE-0991 prevents the loss of cell number (compare Panel D to Panel B).

Figure 2 Fluorescence micrographs of alveolar epithelial cell nuclei on transwell polycarbonate filters. Adherent A549 cell monolayers were exposed to 95% O2 or 21% O2 for 72 hours in the presence or absence of AVE-0991. Monolayers were then fixed with 70% ethanol and were subjected to RNase digestion and propidium iodide staining. Panel A: uniform distribution of control cells. Panel B: A549 cell monolayers exposed to 95% O2 for 72 hours; note the significant loss of cells. Panel C: A549 cell monolayers that received AVE0991 in normoxia. Panel D: A549 cell monolayers exposed to 95% O2 in presence of AVE0991; note preservation of cell number.
Apoptosis inhibitors prevent the hyperoxia-induced albumin flux through alveolar epithelial cell monolayers.
In Figure 3, the pan-caspase inhibitor ZVAD-fmk or the endonuclease inhibitor aurintricarboxylic acid (ATA) prevented the hyperoxia-induced increase in Rhodamine-BSA flux through cultured A549 cell monolayers.
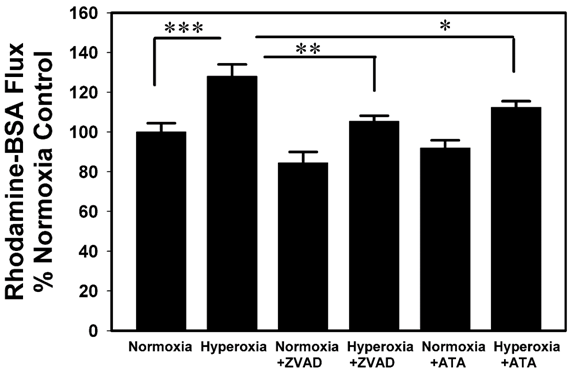
Figure 3 Inhibitors of apoptosis block Rh-BSA flux across A549 cell monolayers in response to 95% Oxygen. A549 cells were cultured on Transwell inserts as described in Figure 1 and Methods, but the cells were exposed to 95% O2 in the presence or absence of the apoptosis inhibitors Z-VAD-fmk (ZVAD, a pan-caspase inhibitor), or the endonuclease inhibitor Aurintricarboxylic acid (ATA). Thereafter, Rh-BSA (1mg/ml) was added into the inner chambers for measurement of Rh-BSA flux as described in Figure 1. Bars are the mean +/- S.E.M.; *** p<0.001, ** p<0.01, * p<0.05 using ANOVA Student-Newman-Keuls test, n=6.
Mas receptor activation prevents hyperoxia-induced alveolar epithelial cell apoptosis
Figure 4 shows that AVE-0991 prevented the induction of nuclear fragmentation, the final stage of apoptosis, in A549 cell monolayers exposed to hyperoxic gas.
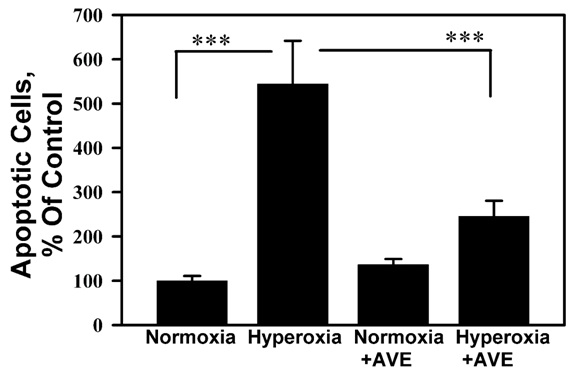
Figure 4 Mas activation inhibits hyperoxia-induced apoptosis in A549 cells. A549 cell monolayers were exposed to 95% O2 in the presence or absence of the mas activator AVE0991. Cells were subjected to propidium iodide (PI) nuclear fragmentation assay (see Methods) after 72 hours exposure. Bars are means +/- S.E.M. normalized as percent of the normoxia control; ***P < 0.001 by ANOVA and Student-Newman-Keuls analysis, n ≥ 6.
Hyperoxic lung injury, and BPD has disturbance of lung fluid balance that may lead to pulmonary edema or fluid overload in lung, as being called in premature X-rays in NICU as “wet lungs”, and diuretics is still a commonly used medicine to help clearing that and drying up the lung. This alveolar fluid overload and airways fluid accumulation is attributed to increased pulmonary permeability and disruption of the epithelial barrier.6
Xu et al. showed that Pulmonary edema secondary to hyperoxic lung injury is secondary to injury to the pulmonary epithelial barrier, with increase in the total water Wet/Dry ratio.24 This Wet/Dry ratio was significant as early as the third day of hyperoxic exposure and increased on fifth then seventh day of hyperoxic exposure. Also there was significant increase in extravascular lung water content, and bronchoalveolar lavage fluid (BALF): serum FD4 “fluorescein isothiocyanate-conjugated dextran 4000 ratio suggesting increased permeability with hyperoxia induced lung injury. In our study, we similarly show that hyperoxia increases the permeability of the epithelial barrier function after 72 hours of oxygen exposure (Figures 1 & 3). Other reports suggest that structure destruction of tight junction proteins occludin and ZO-1 is the mechanism of hyperoxia-induced leakage/pulmonary edema.25,26
Tight junction proteins are necessary for the functional structure of the epithelial barrier. They also play a role in cell signaling, transcription regulation and maintaining epithelial polarity.27,28 Several studies have shown a relationship and cross-talk between the disruption of tight junction proteins and apoptosis. Beeman et al. showed that treating epithelial cells with a claudin-disrupting mimic peptide results in caspase 3 activation and apoptosis. The study also suggests a role of occludin in signaling cell death.29 In an animal study of rat mammary epithelia, apoptosis was found to be preceded by ZO-1 and occludin loss.30 Induction of apoptosis in epithelial kidney, colon, and breast cell lines caused proteolytic cleavage of tight junction proteins in a distinctive manner that correlated with the disruption of the tight junction.31 Notably, occludin, ZO-1 and ZO-2 were found to be fragmented by caspase cleavage and this fragmentation was blocked by caspase and/or metalloproteinase inhibitors. This blockage of fragmentation of these tight junction proteins was associated with preservation of cellular morphology.31 Our data presented here supports these findings where we show that inhibition of apoptosis eliminated the hyperoxia-induced epithelial permeability. This inhibition of apoptosis is presented here using three approaches; the caspase inhibitor ZVAD, the endonuclease inhibitor ATA (Figure 3), and the Mas receptor agonist AVE0991 (Figures 1& 4).
Previous studies from our and other study groups have shown that epithelial apoptosis plays a critical role in lung edema formation. Noradrenaline administration in vivo was shown to be sufficient to invoke the collapse of the alveolar epithelial barrier via induction of apoptosis.9 In animal and cell line acute lung injury models, Fas activation altered the alveolar barrier integrity and function by mechanisms involving caspase-dependent apoptosis.32 In addition, intratracheal instillation of exogenous AngII peptide induced apoptosis and caused alveolar epithelial barrier injury through the AT1 receptor.10 Our data here show a role of the Ang1-7 receptor, Mas, in protection against hyperoxia-induced apoptosis (Figure 4). These emphasize the critical roles of the angiotensin system in the epithelial apoptosis in lung injury, hence epithelial barrier permeability and lung edema formation.
The angiotensin system has been reported to control tight junction proteins localization and distribution.33 Several studies reported that AngII changes ZO-1 expression and cellular distribution in kidney epithelial cells, and that these changes could be blocked by AT1 receptor blockade.34,35 In diabetic retinopathy, AngII also mediated the loss of tight junction proteins in retinal endothelial cells, an effect that could be blocked by angiotensin converting enzyme (ACE) inhibition.36 While the effects of AngII on tight junction proteins in the blood brain barrier (BBB) remain controversial, AngII was shown to increase permeability of the BBB via its action on AT1 receptor.37,38
Hyperoxia has long been known to induce AEC apoptosis.39,40 Apoptosis occurs in the alveolar epithelia of the preterm infants neonatal lungs suffering from respiratory distress and receiving supplemental oxygen and mechanical ventilation,41,42 and in animal models of BPD.43,44 The autocrine angiotensin system is well known to contribute to AEC apoptosis, whereas the pro-apoptotic arm ACE/AngII/AT1 is opposed by the protective ACE2/Ang1-7/Mas axis.14-17,45-50. In our data the Mas receptor activator AVE0991 restored the epithelial barrier integrity and inhibited the hyperoxia-induced AECs apoptosis (Figures 1 & 4). Whether apoptosis is the cause of tight junction loss, or antagonizing apoptosis caused the restoration of tight junction, and the exact mechanisms by which AVE0991 restores the epithelial barrier integrity will be the topics of future studies.
In summary, our study suggests the involvement of the angiotensin system in the pathogenesis of hyperoxia-induced pulmonary edema in BPD and reveals the potential for utilizing AVE0991 or mas receptor activation as a candidate therapy for BPD treatment.
This work was supported by the Department of Pediatrics and Human Development at Michigan State University through start-up funds awarded to Dr. Tarek Mohamed.
The authors declare that there is no conflict of interest.

©2019 Abdul-Hafez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.