Journal of
eISSN: 2376-0060


Research Article Volume 4 Issue 4
1Department of pediatrics, Chonnam National University Medical School and Hospital, Republic of Korea
2College of Veterinary Medicine, Chonbuk National University, Republic of Korea
3National Institute of Animal Science, Republic of Korea
4Department of Pediatrics, KS hospital, Republic of Korea
5Institute of Animal Medicine, Gyeongsang National University, Republic of Korea
6Department of Thoracic and Cardiovascular surgery, Chonnam National University Medical School and Hospital, Republic of Korea
Correspondence: Yu DH, Institute of Animal Medicine, Gyeongsang National University, Jinju 52828, Republic of Korea
Received: July 25, 2017 | Published: August 16, 2017
Citation: Cho HJ, Kim SS, Jung S, Kim S, Ma JS et al. (2017) Effects of Lower Tidal Volume on Ventilator-Induced Diaphragmatic Dysfunction. J Lung Pulm Respir Res 4(4): 00134. DOI: 10.15406/jlprr.2017.04.00134
Purpose: Mechanical ventilation (MV) is one of the most important treatment to achieve sufficient alveolar ventilation in patients with lack of adequate pulmonary gas exchange. This study was performed to evaluate the effects of tidal volume ventilation to diaphragmatic dysfunction by both histological evaluation and the main protease pathway after MV with a murine ventilator induced diaphragmatic dysfunction (VIDD) model.
Materials and Methods: Healthy male C57/BL6 mice (10 – 12 weeks old, 25 – 30 g) were randomly divided into 3 experimental groups: high tidal volume MV for 6 hours (HTV group, n = 6), low tidal volume MV for 6 hours (LTV group, n = 6), and control (Control group, n = 6). Arterial blood gas analysis, examination of diaphragmatic contractile properties, histological evaluation, and biochemical evaluation of main proteolysis pathways were performed in all three groups.
Results: The results of arterial blood gas analysis were comparable among the three groups and all of them were within the normal ranges. Diaphragmatic force production was lower in the HTV group compared to the LTV and control group, respectively. Diaphragmatic force production in the LTV group was higher than that in the HTV group but only slightly reduced compared to the control group. There were no histological differences were found between the groups. No alterations in the level of calpain 1 or 2 were observed among the group, respectively.
Conclusion: Lower tidal volume ventilation partially reduces ventilator-induced diaphragmatic dysfunction. Further studies are needed to determine the mechanism.
Keywords: ventilator-induced diaphragmatic dysfunction; low tidal volume ventilation; murine model
Mechanical ventilation (MV) is one of the most important treatments in patients with inadequate pulmonary gas exchange to achieve sufficient alveolar ventilation. The indications for MV are respiratory failures of various etiologies, including chronic obstructive pulmonary disease, status asthmaticus, and/or heart failure, acute drug overdose, neuromuscular diseases, sepsis, and during the perioperative periods.1 In the USA, more than 300,000 patients receive prolonged MV each year in intensive care units (ICU),2 and it seems like that the number of patients who needs MV is increasing in Korea, however, the exact data is not in the literature. Although MV is a life-saving treatment, prolonged MV results in difficulty in weaning off the mechanical ventilation mainly due to the rapid development of diaphragmatic muscle atrophy and contractile dysfunction. This phenomenon is termed ventilator-induced diaphragmatic dysfunction (VIDD), and is one of the major contributing factors for difficulty in weaning from MV.3,4
Low tidal volume ventilation (LTVV) is one of the important strategy for protection against ventilator-associated lung injury.5 However, there is no evidence that LTVV has a protective effect on the diaphragm, especially in the cases of VIDD. I hypothesized that lower tidal volume venilation has positive effect on diaphragm as it does on lung during MV. Therefore, this study was performed to evaluate the effects of tidal volume by both histological evaluation and the main protease pathway after MV.
Healthy male C57/BL6 mice (10 - 12 weeks old, 25 - 30 g) were randomly divided into three experimental groups: 1) high tidal volume MV for 6 hours (HTV group, n = 6), 2) low tidal volume MV for 6 hours (LTV group, n = 6), and 3) controls (Control group, n = 6).The mice in the control group were anesthetized with intraperitoneal pentobarbital sodium (50 mg/kg body weight) and sacrificed without being mechanically ventilated.
Experimental protocol for MV
All of the mice in three groups were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight). The trachea was incised and a 22-gauge angiocatheter was inserted. To maintain hemodynamic stability, 0.05 ml of Ringer’s lactate solution was infused intraperitoneally every hour. Other general care included bladder expression, ocular lubrication, and passive limb movements. A small animal ventilator (FlexiVent®; SCIREQ Inc., Montreal, QC, Canada) was used for MV with the following ventilator settings: inspired oxygen fraction, 0.21; controlled volume mode; respiratory rate, 150 breaths/min; and positive end-expiratory pressure, 3 – 4 cm H2O. Tidal volume was set at10 µl/mg body weight in the HTV group and 6 µl/mg in the LTV group.
Invasive lung function measurement by forced oscillation technique (FlexiVent®)
The mice in the control group were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight) and sacrificed without being mechanically ventilated. Before sacrifice, their trachea were incised and a 22-gauge angiocatheter were inserted for lung function measurement in all 3 groups. In HTV and LTV groups, lung functions of the mice were measured following completion of 6 hours of MV.
In tracheostomized mice of the three groups, lung function was measured by forced oscillation perturbation (“primewave-8”) in the FlexiVent (SCIREQ) system, including airway resistance [Rn], tissue damping (resistance) [G], tissue elasticity [H], and tissue hysteresivity (tissue damping [G]/[H]). All perturbations were performed until three correct measurements were obtained and the coefficient of determination of 0.95 was the lower limit for accepting a measurement. On average, three measurements were calculated and depicted for each parameter per mouse.
Blood collection
Following completion of lung function measurement in all three groups, blood was collected from the left ventricle for blood gas analysis (i-STAT, Blood Gas Analyzer; Abbot, Hoofddorp, Netherlands) before sacrifice.
Tissue collection
The mice were sacrificed immediately after lung function measurement and blood collection in all three groups. Combined thoracotomy and laparotomy was performed to collect the diaphragm.
Measurement of diaphragm contractile properties
The diaphragms were stimulated by square-wave pulses (Model S48; Grass Instruments, West Warwick, RI). The force-frequency relationship was measured sequentially by stimulating the diaphragms at 10, 20, 30, 50, 60, 80, 100, and 120 Hz for 600 ms with 1 minute interval between the stimulation trains. Fatigability was assessed by measuring the loss of force in response to repeated stimuli over 10 minutes at 30 Hz and 300-ms duration. After assessment of contractile properties, the length and weight of the diaphragm were measured. The cross-sectional area of the diaphragm was calculated by the following equation: weight/ length × tissue density (1.056 g/cm3). The diaphragmatic contractile properties were normalized relative to cross-sectional area and specific force was determined and expressed in Newton’s per square centimeter (N/cm2).
Measurement of inflammation in the diaphragm
To evaluate atrophy of the diaphragmatic muscle, unfixed cryostat sections 7 μm thick were stained with hematoxylin and eosin. The complete area was evaluated for invasion of neutrophils and lymphatic cells by a blinded pathologist using a OLYMPUS BX43 microscope. Images were obtained under the same conditions and visualized using the same lens. The shapes of the muscle fibers were defined accurately on images at 400x magnification.
Measurement of sarcolemmal injury in the diaphragm
The diaphragm muscles were exposed to a fluorescent tracer dye (Procion orange; Sigma, St. Louis, MO) to evaluate the level of acute sarcolemmal injury in diaphragm myofibers. The sarcolemma of normal myofibers is known to be impermeable to Procion orange, and damaged sarcolemmal fibers can be identified by their inability to exclude Procion orange from the cytoplasm.6
Muscle strips from each group of diaphragms were immerged in Procion orange solution for 60 minutes with continuous oxygenation at room temperature. Subsequently, the muscle was rinsed and snap-frozened in isopentane precooled with liquid nitrogen, and then stored at –80°C. Frozen sections (2 μm thick) were viewed by a blinded pathologis under epifluorescence microscopy (ECLIPSE 80i; Nikon, Tokyo, Japan) and the images were saved to computer (Digital Sight DS-U1; Nikon). The numbers of myofibers with clear cytoplasm were determined, and at least 200 myofibers from randomly selected microscopic fields were counted in each tissue section.
Biochemical evaluation
Immunoblotting analysis was performed to evaluate the ratios of the active forms of calpains 1 and 2 to the total levels of each calpain isoform. The total and cleaved forms of caspase 3 were also measured. Diaphragms obtained from each group were lysed by sonication in radioimmunoprecipitation assay (RIPA) buffer containing a protease inhibitor (50 mm Tris, pH 7.5, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mm EDTA). Aliquots of about 30 μg/lane were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 10% gel) and then transferred onto nitrocellulose membranes at 30V, 4°C for 16 hours. After transfer, the membranes were blocked by incubation with 5% skim-milk in TBST (0.1% Tween 20 in Tris-buffered saline, pH 7.4) for 1 hour at room temperature. The membranes were then incubated with specific primary antibodies to the following: calpain 1 (ab 49652 1:1000 dilution in 3% BSA/TBST, 80 kDa; Abcam, Cambridge, MA) and calpain 2 (ab39165 1:1000 dilution with TBST containing 5% skim-milk, 76 kDa; Abcam) at 4°C overnight (Cell Signaling, Danvers, MA). The membranes were then incubated with secondary antibody: anti-mouse (CST, 7076, 1:2000 with TBST containing 5% skim-milk) for calpain 1 and anti-rabbit (CST, 7074, 1:2000 with TBST containing 5% skim-milk) for calpain 2 and GAPDH for 1 hour in the dark. After final washes, the membranes were scanned using an Odyssey™ Infrared Imager (LI-COR® Biosciences, Lincoln, NE).
Statistical analysis
Data are presented as the median (range). General characteristics, blood gas analysis results, and force-frequency response of muscle contractility were analyzed using the Kruskal–Wallis test. In those with significant differences after Kruskal-Wallis test, additional analysis between two groups was performed using the Mann–Whitney test. Differences in muscle contractility force-frequency response and muscle contractility for fatigability between groups were compared using repeated-measures ANOVA. Group and time were considered as fixed variables. Individual animals were considered as a random effect. Statistical analysis was performed using SPSS 21.0. In all analyses, P < 0.05 was taken to indicate statistical significance.
Animal characteristics
The mean body weight of mice was 22.0 (20.0-26.4) g and was not significantly different between groups (Table 1). The mice allocated to the ventilator group were maintained stable for 6 hours of ventilation. No self-respiratory efforts were observed on pressure traces.
HTV (n = 6) |
LTV (n = 6) |
Control (n = 6) |
P-Value |
|
Body weight (g) |
22.0 (21.0-23.0) |
22.0 (20.0-23.0) |
22.5 (22.0-26.4) |
0.273 |
Ventilator |
||||
PIP |
10.7 (10.0-12.6) |
8.5 (8.0-9.9) |
- |
0.006 |
PEEP |
2.9 (2.3-3.0) |
2.9 (2.8-3.1) |
- |
0.873 |
TV |
0.20 (0.19-0.21) |
0.12 (0.120-0.127) |
- |
0.005 |
Table 1 Data are presented as median (range).
HTV, high tidal volume; LTV, low tidal volume; PIP, peak inspiratory pressure; PEEP, positive end-expiratory pressure; TV, tidal volume
Respiratory and hemodynamic monitoring data
During 6 hours of MV, the mean peak inspiratory pressures were 10.7 (10.0-12.6) and 8.59 (8.0-9.9) cmH2O, and the mean positive end-expiratory pressures were 2.9 (2.3-3.0) and 2.9 (2.8-3.1) cmH2O in the HTV group and LTV group, respectively. The mean tidal volumes were 0.20 (0.19-0.21) ml and 0.12 (0.120-0.127) ml in the HTV and LTV groups, respectively. There were significant differences in mean peak inspiratory pressure (P = 0.006) and tidal volume (P = 0.005) between the HTV and LTV groups (Table 1). The heart rate over 6 hours of MV did not differ significantly between HTV and LTV groups (Table 2). The arterial pH, PaO2, PaCO2, and HCO3 were not significantly different between groups (Table 3).
Hour 1 |
Hour 2 |
Hour 3 |
Hour 4 |
Hour 5 |
Hour 6 |
|
HTV |
182.4 (105.4-285.7) |
201.2 (116.8-406.1) |
210.5 (146.5-332.3) |
218.5 (105.5-407.4) |
230.2 (119.4-466.5) |
252.7 (80.5-457.2) |
LTV |
189.3 (101.3-500.1) |
196.6 (135.7-374.3) |
212.4 (133.7-542.5) |
218.2 (154.1-427.3) |
205.5 (154.1-386.6) |
222.2 (164.1-423.1) |
Table 2 Heart rate of the mice in the mechanically ventilated groups
Data are presented as median (range).
HTV, high tidal volume; LTV, low tidal volume
No significant differences were observed across time points between groups.
pH |
PaO2 (mmHg) |
PaCO2 (mmHg) |
HCO3– (mM) |
|
HTV (n = 6) |
7.4 (7.2-7.5) |
58.6 (42.6-76.8) |
22.0 (15.0-26.0) |
20.1 (14-28) |
LTV (n = 6) |
7.4 (7.3-7.4) |
82.2 (76.0-100.7) |
21.0 (19.0-23.0) |
22.4 (22-24) |
Control (n = 6) |
7.4 (7.2-7.5) |
81.5 (76.5-130.0) |
18.0 (15.0-28.0) |
27.8 (22-28) |
Table 3 Arterial blood gas analysis data after 6 hours of mechanical ventilation in ventilated groups and after lung function measurement in the control group
Data are presented as median (range).
HTV: High Tidal Volume; LTV: Low Tidal Volume
No significant differences were observed among the groups.
Invasive lung function measurement by forced oscillation technique (FlexiVent)
In the HTV group, the mean Rn was 0.33 (0.30-0.55) cmH2O·s/ml, G was 4.63 (3.16-6.37) cm H2O/ml and H was 33.5 (24.8-41.2) cm H2O/ml. In the LTV group, the mean Rn was 0.37 (0.31-0.47) cm H2O·s/ml, G was 5.16 (3.94-5.96) cm H2O/ml and H was 40.1 (32.0-43.6) cm H2O/ml. In the control group, the mean Rn was 0.32 (0.23-0.41) cm H2O·s/ml, G was 4.35 (3.14-5.35) cm H2O/ml and H was 28.8 (24.8-44.6) cm H2O/ml. There were no significant differences in Rn, G and H among the three groups (Table 4).
Airway resistance (RN), cmH2O·s/ml |
Tissue damping (G), cm H2O/ml |
Tissue elasticity (H), cm H2O/ml |
|
HTV (n = 6) |
0.33 (0.30-0.55) |
4.63 (3.16-6.37) |
33.5 (24.8-41.2) |
LTV (n = 6) |
0.37 (0.31-0.47) |
5.16 (3.94-5.96) |
40.1 (32.0-43.6) |
Control (n = 6) |
0.32 (0.23-0.41) |
4.35 (3.14-5.35) |
28.8 (24.8-44.6) |
P-value |
0.343 |
0.158 |
0.087 |
Table 4 Invasive lung function measurement using the forced oscillation technique (FlexiVent) in all three groups
Data are presented as median (range).
HTV, high tidal volume; LTV, low tidal volume
No significant differences were observed among the groups.
Isometric contractile properties of the diaphragm
High tidal volume ventilation for 6 hours resulted in significant decreases in diaphragmatic force production compared to the LTV group at stimulation frequencies of 10 Hz (P = 0.01), 20 Hz (P = 0.016), 30 Hz (P = 0.016), 50 Hz (P = 0.037), 60 Hz (P = 0.045), and 80 Hz (P = 0.037). In addition, high tidal volume ventilation for 6 hours resulted in significant decreases in diaphragmatic force production compared to control at all stimulation frequencies examined (P < 0.05) (Figure 1). However, no significant differences in diaphragmatic force production were observed between the LTV and control groups. In addition, during repetitive trains of electrical stimulation for muscle fatigability assessment, diaphragm specific force in the HTV group remained significantly lower than those in the LTV and control groups (P < 0.05) (Figure 2).
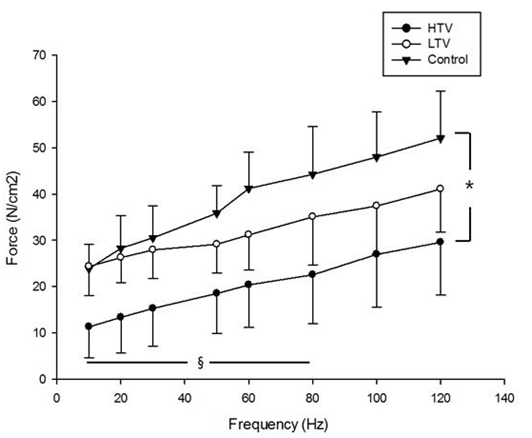
Figure 1 Force-frequency curves of the diaphragm in three group: after 6 hours of mechanical ventilation with a tidal volume of 10 µl/mg (HTV), after 6 hours of mechanical ventilation with a tidal volume of 6 µl/mg (LTV), and in the control group. HV group resulted in significant decreases in diaphragmatic force production compared to the LTV group at stimulation frequencies of 10, 20, 30, 50, 60, and 80 Hz. Also, HTV group resulted in significant decreases in diaphragmatic force production compared to control at all stimulation frequencies examined (P < 0.05). No significant differences in diaphragmatic force production were observed between the LTV and control groups. Values are means ± SE. *P < 0.05, HTV vs. control, § P < 0.05, HTV vs. LTV.
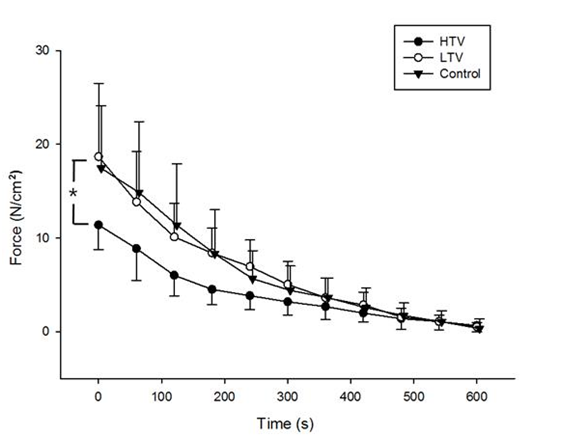
Figure 2 Absolute force production of the diaphragm during the fatigue test after 6 hours of mechanical ventilation with a tidal volume of 10 µl/mg (HTV) and after 6 hours of mechanical ventilation with a tidal volume of 6 µl/mg (LTV), and in the control group. Diaphragm specific force in the HTV group remained significantly lower than those in the LTV and control groups (P < 0.05). Values are means ± SE. *P < 0.05, HTV vs. LTV and control groups.
Histological findings of inflammation in the diaphragm
There was no detectable invasion of neutrophilic or lymphocytic cells and no endomysial fibrosis or atrophy around diaphragmatic muscle fibers in any of the three groups as determined by histological examination of diaphragmatic muscle 7 μm thick (Figure 3). In addition, no atrophy was seen in either the HTV or LTV group.
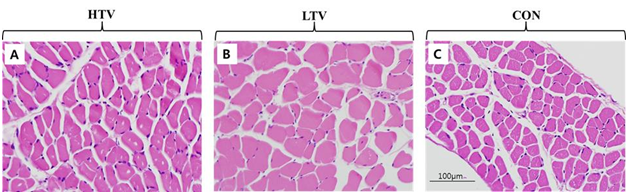
Figure 3 Histologic evaluation of inflammation and atrophy in the diaphragm. Muscle sections of each group were stained with hematoxylin and eosin. Images (400×) of each muscle section were shown without significant difference between groups. A. High tidal volume ventilation (HTV) group. B. Low tidal volume ventilation (LTV) group. C. Control (Con) group.
Quantification of sarcolemmal injury in the diaphragm
The degrees of intracellular Procion orange dye uptake by diaphragmatic muscle in each group are shown in Figure 4. There was a small degree of uptake after 6 hours of MV at 10 μl/mg (HTV) and 6 μl/mg (LTV). On the other hand, there was no intracellular uptake of Procion orange in the control group (Figure 4). However, the mean values did not differ significantly among the groups.
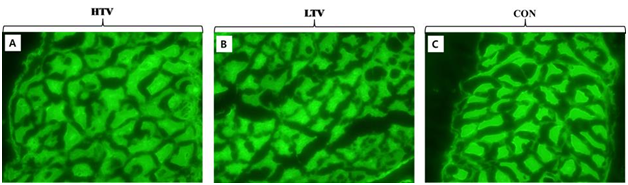
Figure 4 Evaluation of sarcolemmal injury in the diaphragmatic muscle. A small degree of Procion orange dye was taken up intracellularly by myofibers in ventilated groups. However, the mean values did not differ significantly among the three groups, indicating no diaphragm sarcolemmal injury. A. High tidal volume ventilation (HTV) group. B. Low tidal volume ventilation (LTV) group. C. Control (Con) group.
Biochemical evaluation
There were no significant differences in concentrations of cleaved calpain 1 or 2 isoforms among the groups (Figure 5).
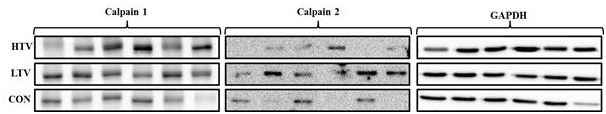
Figure 5 Expression of calpain isoforms in the diaphragm in all three groups: 10 µl/mg tidal volume ventilation for 6 hours (HTV), 6 µl/mg tidal volume ventilation for 6 hours (LTV), and control. Representative immunoblots were obtained from diaphragm tissue of all three groups. There were no significant differences in concentration of calpain 1 and 2 isoform in all 3 groups.
Mechanical ventilation (MV) is a life-saving treatment strategy in patients with acute or chronic inadequate pulmonary gas exchange. However, the imbalance between ventilator and patient created by an increased respiratory load and decreased respiratory muscle movement results in prolonged ventilator dependence.7–9 The decrement of respiratory muscle performance has been described by decreased maximal transdiaphragmatic pressure in patients under MV.8 It has been suggested that prolonged ventilation not only contributes to decreased diaphragmatic performance but also induce respiratory muscle and diaphragmatic weakness.
There have been a number of studies over the past 35 years regarding the phenomenon of diaphragmatic dysfunction induced by MV, so-called VIDD. The first animal study of this issue was reported in 1994, and further such studies have since been performed.1 The first prospective study indicated that controlled MV resulted in diaphragmatic muscle atrophy and contractile dysfunction in rats; this study showed that controlled MV for 48 hours without spontaneous breathing results in a significant decrease in diaphragm muscle mass and also a significant reduction in maximal diaphragmatic specific force production.10 Anzueto et al.11 reported that prolonged MV in healthy baboons showed decreased maximal transdiaphragmatic pressure and diaphragmatic endurance in vivo.11
Levin et al.12 reported that prolonged MV also results in diaphragmatic muscle atrophy in humans.12 They obtained biopsy specimens from diaphragm muscle in brain-dead organ donors who had complete diaphragmatic inactivity and MV for 18 – 69 hours, and reported marked atrophy of diaphragm muscle fibers, consistent with the findings of previous animal studies.12 Significant atrophy of diaphragm muscle fibers after prolonged MV has been reported in various species, including mice, rats, rabbits, and pigs.13–16 Mrozek et al.15 recently reported VIDD in a mouse model in which 6 hours of MV resulted in significant reduction of diaphragmatic specific force.15 However, they did not show any histological findings of atrophy, in contrast to several other animal and human studies.12,14,17,18
Among the various ventilator strategies, LTVV is an important concept of lung protective ventilation. The rationale of this strategy is that smaller tidal volumes are less likely to generate alveolar over distension and consequently less likely to cause ventilator-induced lung injury.12,19 In this present study, we postulated that LTVV might have a protective effect on diaphragm function as it shows lung protection. Accordingly, we expected better contractility and less fatigability in LTVV mice compared to conventionally ventilated controls. The results of my investigation confirmed that LTVV in mice resulted in a significantly smaller degree of reduction in diaphragmatic specific force compared to conventional ventilation.
Although both atrophy and sarcolemmal injury were reported in other animals, we did not observe either finding in mice, as reported previously by Mrozek et al.15 On preliminary study, we ventilated mice for 7 or 8 hours, but this could not maintain hemodynamic stability; therefore further studies are required to determine an adequate ventilation period to observe atrophy and sarcolemmal injury in mice. Multiple mechanisms are involved in VIDD, including increments of reactive oxygen species, mitochondrial dysfunction, inhibition of the insulin-like growth factor pathway, and activation of various proteolytic systems, such as calpains and caspase 3.5
Calpains 1 and 2 are ubiquitous non-lysosomal proteases, and are unregulated early in the time course of VIDD.6–8 The calpains are involved in regulation of the cell cycle, apoptosis, and cytoskeletal reorganization, and also play roles in muscle wasting by initiating myofibrillar disassembly, which causes the myofibrillar proteins to become susceptible to further degradation by the proteasome.21,22 All of these mechanisms result in muscle atrophy, injury of the diaphragmatic muscle, and promote loss of diaphragmatic force-generating capacity.9–11 To explore the mechanism of VIDD in this murine model and to determine the differences between higher and lower tidal volume ventilation, I measured the levels of total calpains 1 and 2 in the diaphragms of these mice. Mrozek et al.15 reported that calpain 1 and 2 levels were not altered in the mechanically ventilated diaphragm after 6 hours compared to a control group, despite observation of a major reduction in specific force production.
There have been many attempts to reduce diaphragmatic muscle weakness or VIDD by adjusting ventilator strategy. Sassoon et al. reported that assisted-control mode of MV reduce VIDD in rabbits.23 Sasson et al.24 also reported that positive end-expiratory airway pressure does not aggravate VIDD in rabbits.24 Schellekens et al.25 reported that hypercapnea partly protects the diaphragm against the adverse effects of MV in a rat VIDD model. This study had the limitation that although mice ventilated at higher tidal volume had greater diaphragm weakness than those ventilated at lower tidal volume or controls, there were no other differences in the results of pulmonary function test, histological evaluation, or biochemical evaluation between groups. The duration of ventilation used in the present study may have been too short to reveal any such differences.
There have been many attempts to reduce diaphragmatic muscle weakness or VIDD by adjusting ventilator strategy. Although this is a preliminary, this is the first attempt to reduce VIDD by lessen lung stretch. In conclusion, in this murine VIDD model lower tidal volume ventilation had a partial protective effect on the diaphragm dysfunction and further studies are needed
This study was supported by a grant (CRI15012-1) from the Chonnam National University Hospital Research Institute of Clinical Medicine.
This work was supported by a grant from NIH (R01 HL-47125).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.

©2017 Cho, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Tuberculosis Day (March 24) provides the opportunity to raise awareness about TB-related
problems and solutions and to support worldwide TB-control efforts. While great strides have been made to control and cure TB, people
still get sick and die from this disease in our country. On this event, we request researchers to spread more information and awareness
on this by their article submissions towards our JLPRR. For this we are rendering 25% partial waiver for articles submitted on or before
March 24th.
World Tuberculosis Day (March 24) provides the opportunity to raise awareness about TB-related
problems and solutions and to support worldwide TB-control efforts. While great strides have been made to control and cure TB, people
still get sick and die from this disease in our country. On this event, we request researchers to spread more information and awareness
on this by their article submissions towards our JLPRR. For this we are rendering 25% partial waiver for articles submitted on or before
March 24th.