Journal of
eISSN: 2376-0060


Research Article Volume 1 Issue 3
1Department of Psychiatry and Psychotherapy, Friedrich-Alexander University Erlangen-Nuremberg, Germany
2Medical Director emeritus, Department of Pneumology, University Hospital Freiburg, Germany
3Paediatric Clinic, Evangelic Hospital Hamm gGmbH, Germany
4Group Practice and Centre for Allergy, Respiratory and Sleep Medicine at Red Cross Maingau Hospital, Germany
Correspondence: Peter Kardos, Group Practice and Centre for Allergy, Respiratory and Sleep Medicine at Red Cross Maingau Hospital, Frankfurt am Main, Germany, Tel 4969553611, Fax 4969594781
Received: October 05, 2014 | Published: October 27, 2014
Citation: Lehrl S, Matthys H, Kamin W, Kardos P (2014) The BSS - A Valid Clinical Instrument to Measure the Severity of Acute Bronchitis. J Lung Pulm Respir Res 1(3): 00016. DOI: 10.15406/jlprr.2014.01.00016
Introduction: Several validated clinical psychometric procedures have been developed for the severity measurement of chronic lung diseases such as COPD. In contrast, no validated tool for acute bronchitis was available. A valuable tool might be the “Bronchitis Severity Scale” BSS, a short standardized observer assessment-scale. But does it fulfil psychometric criteria of validity?
Patients and methods: The relevance of the score items of BSS has been evaluated, and the data of 2,033 patients (1-92years old; 39% males) with acute bronchitis out of eight longitudinal studies have been analyzed.
Results: A high content validity was found. Factor analyses indicate that the construct of one single factor of severity is justifiable. The construct validity increases about 68.5% when a second independent factor is adopted. The contents are centred on “cough” and “sputum”, the main symptoms and complaints in acute bronchitis. Substantial correlations between BSS scores and patient’s self-assessment as well as reduced everyday activities, emotions, and health support the concurrent validity. Evidences of predictive validity are given in 16 publications.
Conclusion: The BSS composite and its subscales are valid clinical measures for recording the severity of acute bronchitis both at initial diagnosis and treatment control whose responsiveness to change are also confirmed.
Keywords: bronchitis severity score, coughing, outcome assessment, severity of illness index, sputu
AB, acute bronchitis; RTI, respiratory tract infection; COPD, chronic obstructive pulmonary disease; SGRQ, St. george’s respiratory questionnaire; CRQ, chronic respiratory questionnaire; AQ20, airways questionnaire 20; BSS, bronchitis severity scale; ABSS, acute bronchitis severity score; HIV, human immunodeficiency virus
For various reasons, standardized psychometric clinical measures of the severity of acute bronchitis (AB) are needed. They help to communicate more exactly on the degree of symptoms and the syndrome, respectively, and they contribute to objective assessment of clinical courses. In the clinic, it is not only necessary to measure outcomes of treatment regarding the intervention process, it is also essential to measure the extent to which the patients feel the treatment has influenced their condition and quality of life.1 In this context, patient related rather than surrogate clinical outcome measures are valid, which should be based on both physicians and patient assessment. As will be shown subsequently, the psychometric clinical measures in the field of chronic lung diseases are more disseminated and more developed than those for AB, also referred to as acute respiratory tract infection RTI.2 Measuring the severity of AB at a high psychometric level, however, seems to be overdue because this clinical syndrome, with cough being its strongest independent predictor, is relevant if only for its frequency.3 To assess severity and outcome in chronic diseases, readily available, valid psychometric procedures are applied and analysed (e.g. CAT, CCQ, mMRC, ACQ, HADS, Beck Depression Inventory etc.). An attempt for assessment of AB will be performed subsequently. Beforehand, a short overview is to be given on the situation of psychometric measures for chronic lung diseases and AB in order to recognize the gap in the latter field. Most of the survey is orientated on information drawn from PubMed.
Clinical psychometric measures for chronic lung diseases
For more than 20years, psychometric clinical measures have been administered in order to assess the severity and course of chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD). Up to now, the best-known and most frequently administered disease-specific tools among the pneumologists are the Chronic Respiratory Questionnaire (CRQ)4 and the St. George’s Respiratory Questionnaire (SGRQ).5
The CRQ covers aspects of disability in patients with chronic lung disease. It is available as an interviewer and a self-administered instrument that includes 20 items across four domains: dyspnoea, fatigue, emotional function, and mastery.
The SGRQ consists of 50 items that are designed to measure impact on overall health, daily life, and perceived well-being in patients with obstructive airway disease. Several more practical modifications were developed, e. g. the SGRQ-A, published in 2000,6 in which the symptom-reporting component covered a period of 1month instead of 1year as the original SGRQ required. In 2006, a 40-item-version (SGRQ-C) was published that seems to be equally valid.7 In order to assess the course of exacerbations, daily measurements were necessary. Therefore, the “Exacerbations of Chronic Pulmonary Disease Tool” (EXACT-PRO) was developed and presented in 2011.8 EXACT is a patient-reported outcome diary comprising 14items, which is to be completed by the patient on an electronic diary every evening before bedtime. Additionally, further disease-specific questionnaires have been developed for patients with chronic respiratory diseases such as the Airways Questionnaire 20 (AQ20),9 which consists of 20items with yes/no responses and takes 2minutes to complete and score. Even more simple and shorter is the linear analogue scale questionnaire “QoL scale” to measure disease-specific health-related quality of life in elderly patients with COPD.10 In 2005, Au et al.,11 published the Chronic Bronchitis Symptoms Assessment Scale (CBSAS) comprising 15 scored items (16 items total). This likewise easy-to-administer assessment scale measures the symptom severity among patients with chronic bronchitis once daily or once weekly.
Even the oldest of the presented psychometric clinical instruments for the severity of symptoms of chronic respiratory diseases has been investigated for its validity. Validity is the final decisive criterion for the scientific and practical value of a measure12 as it deals with the question to which extent a tool measures what is pretended to be measured. In this case, it is the severity of symptoms of chronic respiratory diseases. Additionally, since introduction of the above presented clinical measures, the trend in the respiratory field was to make their administration more practical, more disease specific and, if needed, to shorten the periods the assessments refer to.
Clinical psychometric measures for acute bronchitis
An extensive PubMed search revealed only two measures, the “Bronchitis Severity Scale” (BSS) and the “Acute Bronchitis Severity Score” (ABSS), which are both described subsequently with the first being nearly as old as the CRQ and the SGRQ for chronic bronchitis. On the other hand, the ABSS is a relatively young development.
The instrument BSS: It is an observer clinical assessment scale, which results in a Bronchitis Symptom Score designed to indicate the severity of AB. The assessments refer to the current state of a patient at a point of time, e.g. at baseline and under treatment. The BSS should be applied by physicians in presence of the patient, since it contains both patient-reported and physician-assessed items.
The BSS comprises the following five symptoms typical for AB: cough, sputum, rales on auscultation, chest pain on coughing, and dyspnoea. These symptoms are each assessed according to a 5-point Likert scale: 0=absent, 1=mild, 2=moderate, 3=severe and 4=very severe. The points allocated to each of the symptoms are added to make the total score. This can therefore vary between 0 and 20 points and should show a one-dimensional degree of severity in the clinical signs of AB. The score can be applied to children and adults, regardless of the sex of the patients.
The BSS in the literature: The symptoms and findings assessed in the BSS were first described in 1996 by Haidvogl et al.13 and Dome et al.14 Later on, in 1999 and 2002, Blochin et al.15 and Golovatiouk et al.,16 used the full scale, but the term “BSS” was introduced in the scientific literature in 2003 by Matthys et al.17 and has since been used in many further publications e.g.18–29 Recently, a review of all trials employing the BSS until April 2012 was published.30 Moreover, Kardos and colleagues reported of the BSS as a current example for the use of a composite scale.31
The instrument ABSS: This scoring system for the severity of AB was presented in 2007.32 It is a questionnaire for the self-assessment of patients aged 18years and older; it was developed to assess clinical response to therapy in a high human immunodeficiency virus (HIV) prevalence setting. Patients have to assess the degree to which five symptoms have bothered them within the past 24hours. The symptoms are: Overall severity of health, day cough, night cough, limited daily activity, and fever. The degrees of severity vary between 0 (mild, seldom, or none) and 4 points (very serious, >20times/(day resp. night,) or very severe).
As a principal component analysis32 shows, the loadings of the five items on the first factor are so high that they justify the assumption of a one-dimensional severity of AB. Therefore, the points of the five symptoms can be summed up to make the total score. This can vary between 0 and 20points. The full scale is published in.32
Both the ABSS and BSS are clinical scoring systems for the severity of AB. Although the psychometric properties of the ABSS are explicitly investigated and those of the BSS are not, the ABSS has only been validated in a high HIV-sero prevalence setting. Moreover, as the symptom fever should take little account especially for the indication “simple” AB and should therefore better serve as a diagnostic exclusion criterion, its use as a score item should be seen critically.
Although the BSS has been applied by far earlier and in more clinical studies than the ABSS, formally, no methodological validation data have been published up to now. Therefore, the data of all available studies with the outcome BSS are analysed here to establish the BSS validity.
The objective is to find an answer to the question whether the statistical analyses of the available studies applying the BSS indicate that this measure is as valid as is expected for psychometric tests. In particular, the validity of the BSS refers to the degree of severity of AB.
Studies, patients and study designs
The analyses were based on the data concerning the five BSS items derived from all placebo-controlled, randomized, double-blind, clinical studies in the indication AB, published until April 2012 or earlier, for which a complete data set was available. All eligible studies were conducted with EPs 7630.17–24 Planning, execution and analysis of these clinical trials were carried out in accordance with the national regulations, the Declaration of Helsinki and the ICH-Guideline for Good Clinical Practice. The study protocols and other required study documents were submitted to the respective independent ethics committee and regulatory authorities for approval. All participants in the studies gave their informed consent.
One study was subdivided into two sections (study17), because one part was performed in Germany with German doctors and patients and the other in Ukraine with Ukrainian doctors and patients. Possibly the different backgrounds of history and native language could exert different influences on the results. For this reason, the following statistical analyses are based on eight studies (Table 1). In these clinical trials, the BSS was applied in each study participant three times over seven days (visits 1, 2 and 3). After the first visit, the patients were treated with either an active substance (verum) or a placebo preparation.
| Study Designation |
Participants |
Number (Age Range) |
Treatment Groups* |
Particular Feature |
Publication |
|---|---|---|---|---|---|
1 |
Children and adolescents with acute bronchitis (1 to 18 years old) |
200 (1-18 yrs) |
2 |
|
Kamin et al. [18] |
2 |
399 (6-18 yrs)
|
4 |
Dose-finding study |
Kamin et al. [19] |
|
3 |
220 (1-18 yrs) |
2 |
|
Kamin et al. [20] |
|
4 |
Adults with acute bronchitis (18 to 92 years old) |
405 |
4 |
Dose-finding study |
Matthys et al. [21] |
5 |
124 |
2 |
|
Chuchalin et al. [22] |
|
6a** |
262 |
2 |
|
Matthys et al. [17] |
|
6b*** |
206 |
2 |
|
Matthys et al. [17] |
|
7 |
217 |
2 |
|
Matthys and Heger [23]; Matthys and Funk [24] |
|
Participants in Total |
2.033 |
|
|
|
|
Table 1 Overview of the studies underlying the statistical analyses
*Always one placebo group; at least 1 verum group
**Part of study 6 performed in Germany with German doctors and patients
***Part of study 6 performed in Ukraine with Ukrainian doctors and patients
Methods
Primarily, the classical test theory comprises three types of validity: content validity, construct validity and criterion validity.33 Criterion validity is subdivided into concurrent validity and predictive (prognostic) validity. All these different aspects of validity shall be examined by analysing the BSS and its development as well as the data produced with its application.
Content validity
The content validity investigates whether the items included in the scale embrace the expert knowledge about AB. For a valid application, it is also relevant to establish whether the items can be assessed in a valid manner by users treating patients with AB.
Construct validity
When determining constructs validity, the important question is how well a measurement instrument is in agreement with the theory from which its construction was derived. In the literature, AB is presented as a syndrome, i.e. a collection of symptoms and clinical findings. Each of them may occur with different degrees of severity. Accordingly, the measurement of AB severity by means of the five bronchitis-specific items of the BSS, are to be added to a total score. This implies the construct that AB has a univariate characteristic with different degrees of severity.
The statistical analysis of the relationships between the BSS items allows establishing of whether it is more appropriate to assume that AB has a single characteristic with different degrees of severity or whether it is split into two or more mutually independent characteristics, each with its own degree of severity. For instance, a subdivision of the severity of AB into the two subscores “coughing complex” and “sputum” has been emphasized by Timmer et al.,34 in a meta-analysis by the Cochrane Collaboration.
The factor analysis model lends itself to structural analysis. The analyses were based on individual data for the five BSS items which was derived from eight studies (Table 1) with three visit days each in patients receiving either placebo or verum. The remarkable similarity in the study designs allows analysing and stating the individual patient data. Since, in terms of the measurement, the patients were not given any study medication prior to the first visit, no distinction was made within the factor analyses between the patients with subsequent placebo treatment and those with verum treatment. This distinction was made only for the second and third visits. In this manner, 40 factor analyses (principal component analyses35) were performed: 8 of the 8 studies on the 1st visit and 32 of the 8 studies x 2 treatment groups’ (placebo and verum)x2 visits (i.e. 2nd and 3rd visits).
Criterion validity
Which are the criteria in the available studies that can be used to examine to what extent the items of the BSS measure indeed what they are supposed to assess? Several such criteria exist, some of them concerning the concurrent validity, others the predictive validity.
Concurrent validity: In addition to the BSS, several self-assessments were documented in the studies, which can be used to examine the concurrent validity. Particularly, “general state of health” and “vitality” (energy) in the longer term had been evaluated by means of the “SF-12” questionnaire36 in various studies.17,21–24 It can be reasonably expected that these parameters are diminished by AB.
The same is hypothesized for the EuroQol instrument EQ-5D scale for self-assessed health status37 that was administered in some of the studies.17–24 It records the current condition by means of the five dimensions concerning mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (three levels: 1=no problem to 3=extreme problems). In addition, the EQ-5D contains a visual analogue scale (EQ VAS) from 0 (worst imaginable health state) to 100 mm (best imaginable health state) by means of which the patient scores his/her current health condition. All these aspects should correlate statistically significantly with BSS.
Predictive validity
It is given when predictions about future events are possible on the basis of the measurement findings. Two predictions for testing the validity of the BSS can be formulated as follows
The 7 studies were analysed by statistical methods as follows:
Content validity
Several facts indicate a high content validity, as can be checked by referring to the specialist literature.
It appears that there is a broad consensus that the five symptoms of the BSS rank among the principal symptoms of AB. Matthys et al.,25 Chuchalin et al.,22 as well as Matthys et al.,23 who go back to Williamson38 and Macfarlane et al.,39 evaluate them explicitly as “the five most important symptoms” of AB.
A further important indication that the symptoms comprised in the BSS are recognized as elements of AB in the professional respiratory community is provided by the fact that several of the publications in which the BSS was described were peer-reviewed and thereby accepted for publication. Finally, the BSS was brought to the notice of the international scientific community by over a dozen publications13–17,19–29,34 and it was not rejected. All these arguments speak for a high content validity of the BSS.
Whether the BSS user, especially doctors without training in respiratory medicine, can determine the severity of AB symptoms using the scale in a manner that complies with specialist knowledge is decisive for valid application. The BSS was designed for use in primary care, since the vast majority of AB patients will be treated by general practitioners. No health care system in the world has or needs the capacity to treat AB by specialists.
The BSS comprises five typical items of AB (cough, sputum, rales on auscultation, chest pain on coughing, dyspnoea) familiar to general practitioners. These are to be assessed with regard to the applicability of the five specified levels of severity – including “absent”. Because of the combination of objective and subjective items that constitute the BSS, the assessment is based on the general practitioner’s evaluation in conjunction with the subjective feedback of the patient. The medical user of the BSS can be expected to cope reliably with both content assessment and severity assessment.
Construct validity
Acute bronchitis as a two-dimensional construct: The factor analyses resulted in two group factors which varied independently from each other (Table 2) (Table 3). On the first visit on which the random sample was most comprehensive (verum patients + placebo patients) and on which none of the considered artefacts was to be expected, both group factors together covered with 58.4% more common variance than the 1st extracted factor (34.7%) which would have been accepted as sufficient in a one-dimensional concept of AB severity (Table 4). Construct validity increases correspondingly, i.e. for 68.5% (=100x(58.4–34.7)/34.7), in the case of the two-factor solution as compared to the one-factor solution.
|
|
Children and Adolescents |
Adults |
||||||
|---|---|---|---|---|---|---|---|---|
Component |
Study 1 |
Study 2 |
Study 3 |
Study 4 |
Study 5 |
Study 6a |
Study 6b |
Study 7 |
1 |
2.10 |
1.42 |
1.83 |
1.57 |
1.69 |
1.43 |
1.85 |
2.05 |
2 |
1.06 |
1.19 |
1.31 |
1.12 |
1.19 |
1.15 |
1.19 |
1.06 |
3 |
0.82 |
1.04 |
0.81 |
0.92 |
0.84 |
1.02 |
0.88 |
0.88 |
4 |
0.60 |
0.79 |
0.63 |
0.75 |
0.71 |
0.77 |
0.61 |
0.59 |
5 |
0.43 |
0.56 |
0.42 |
0.57 |
0.57 |
0.64 |
0.48 |
0.43 |
Table 2 Eigen values of the measurements at the 1st visit (placebo and verum patients together). Bold print: Eigen values > 1.00
Visit |
1 General Factor |
2 Group Factors |
3 Group Factors |
1 (Placebo & Verum) |
- |
6 |
2 |
2 (Placebo) |
1 |
7 |
- |
2 (Verum) |
- |
8 |
- |
3 (Placebo) |
4 |
4 |
- |
3 (Verum) |
5 |
3 |
- |
Total |
10 |
28 |
2 |
Table 3 Overview of the number m of the factorial solutions with Eigen values > 1.00 in all studies at all visits. From visit 2 on separated according to placebo and verum patients.
|
|
Children and Adolescents |
Adults |
||||||
|---|---|---|---|---|---|---|---|---|
Component |
Study 1 |
Study 2 |
Study 3 |
Study 4 |
Study 5 |
Study 6a |
Study 6b |
Study 7 |
Coughing Complex |
30.1 |
27.5 |
34.0 |
26.1 |
32.0 |
24.0 |
36.3 |
38.7 |
Sputum |
33.0 |
26.1 |
25.5 |
34.1 |
25.5 |
27.4 |
24.5 |
23.5 |
Both Factors Together |
63.1 |
53.6 |
59.5 |
60.2 |
57.5 |
51.4 |
60.8 |
61.2 |
Table 4 Common variance of the first and second factor (after factor rotation) on the first day of visit for verum patients and placebo patients together.
A two-dimensional construct centred round “cough” and “sputum”: How are the two group factors to be interpreted with respect to their content? Responses were given by the loadings of the five BSS items on the two factors determined (principal component analysis with varimax rotation40). The first factor can be designated as “coughing complex” (Table 5), the second as “sputum” (Table 6). The independence of both factors means that a certain degree of severity of the “coughing complex” does not imply a corresponding severity of the sputum. Over the eight-day period from the 1st visit to the 3rd visit, too, they showed a different course (Figure 1).
| Children |
Adults |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Study 1 |
Study 2 |
Study 3 |
Study 4 |
Study 5 |
Study 6a Germany |
Study 6b Ukraine |
Study 7 |
Cough |
906 |
831 |
728 |
659 |
563 |
413 |
731 |
624 |
Sputum Production |
-141 |
-069 |
079 |
223 |
-173 |
-323 |
-221 |
-016 |
Rales at Auscultation |
704 |
773 |
631 |
840 |
272 |
061 |
178 |
-709 |
Chest Pain on Coughing |
408 |
171 |
725 |
301 |
814 |
683 |
741 |
728 |
Dyspnea |
-056 |
-150 |
497 |
166 |
717 |
675 |
807 |
-715 |
Common Variance |
30.1 |
27.5 |
34.0 |
26.1 |
32.0 |
24.0 |
36.3 |
38.7 |
Table 5 Factor “coughing complex” for the bifactorial solutions rotated according to the varimax criterion (loadings a x 1.000).
| Children |
Adults |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Study 1 |
Study 2 |
Study 3 |
Study 4 |
Study 5 |
Study 6a Germany |
Study 6b Ukraine |
Study 7 |
Cough |
077 |
-075 |
360 |
-302 |
413 |
659 |
303 |
019 |
Sputum Production |
760 |
498 |
891 |
815 |
843 |
706 |
710 |
935 |
Rales at Auscultation |
-350 |
-028 |
-146 |
077 |
565 |
617 |
690 |
394 |
Chest Pain on Coughing |
-670 |
-628 |
-169 |
-570 |
-217 |
194 |
-387 |
-297 |
Dyspnea |
-703 |
-787 |
-679 |
-537 |
172 |
-151 |
-045 |
-245 |
Common Variance |
33.0 |
26.1 |
25.5 |
34.1 |
25.5 |
27.4 |
24.5 |
23.5 |
Table 6 Factor “sputum” for the bifactorial solutions rotated according to the varimax criterion.
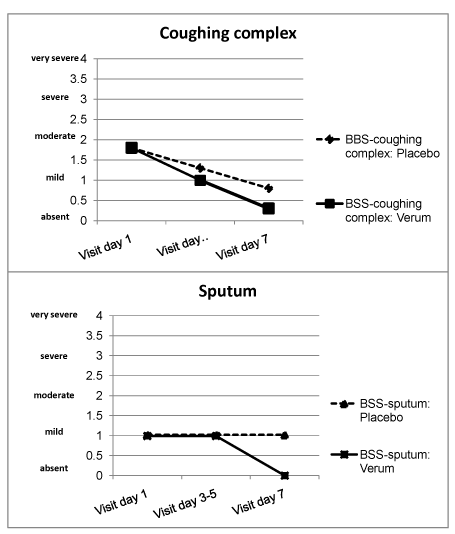
Figure 1 Courses of medians for severity of coughing complex (top) and sputum (bottom) in all patients with placebo (n = 774) and verum treatment (n = 1,181).
Criterion validity
Doctor and patient correlation at item level: In four studies with adults (Studies 5 to 7), patient self-assessments of the three symptoms “cough”, “sputum” and “chest pain on coughing” were entered daily into the patient diary. The self-assessment of the severity of these three symptoms should correlate much more closely with the observer assessments of the same symptoms by the doctor than with the other symptom-specific items for AB.
At the first visit, the correlations (Pearson’s r x 100) between patient’s and doctor’s assessment of the same three symptoms varied in the range of 32 to 95. The medians of the four studies were 87, 67, 95, 67 (here and subsequently in this order: study 5, 6a, 6b, and 7). At the second visit the range of correlation was 44 to 75 (medians: 70, 58, 74, 69), and at the third visit 70 to 97 (medians: 87, 81, 85, 91).
The assessments of the three symptoms in the patients’ diaries have been compared to the different symptoms evaluated by the doctor, e.g. coughing by patient and sputum by doctor. There have been given 12 combinations the correlations of which scattering between -49 and 46 at the first visit, at the following visits from -11 to 57 and 01 to 59. The medians from the first to the third visit were 15.5; 5; 8; -4.5, afterwards 29; 20.5; 38; 27 and finally 41; 29.5; 32 and 34.5.
The correlations between the different symptoms assessed in the patient’s diary reached the same order as those between the different symptoms evaluated by the physician. This holds also true for the correlations of different symptoms according to the physician’s judgment.
BSS and patient quality of life: In the studies 4 and 5 (Table 1), the criteria variables “general health” and “vitality” were measured by SF-12 and in the studies 1 and 2 (Table 1) the “acute health”, state of emotion and everyday activity by EQ-5D. Table 7 contains the correlations with BSS on the 1st and 3rd visit.
|
|
Visit 1 |
Visit 3 |
||||
|---|---|---|---|---|---|---|
|
BSS Coughing Complex |
BSS Sputum |
BSS Total |
BSS Coughing Complex |
BSS Sputum |
BSS Total |
SF-12: General Health |
23* -26* |
-18* -06 |
14* -26 |
55* 59* |
22* 48* |
53* 61* |
SF-12: Vitality |
-02 -17* |
01 00 |
-02 -15* |
46* 30* |
18* 07 |
43* 27* |
EQ-5D: Mobility |
32 07 |
-18 -05 |
22 05 |
26 48* |
09 24* |
26 47* |
EQ-5D: Self-care |
73* 27* |
07 -09 |
79* 21* |
66* 73* |
07 36* |
61* 72* |
EQ-5D: Activities |
54* 34* |
07 01 |
60* 32* |
74* 56* |
24 36* |
72* 57* |
EQ-5D: Pain & Discomfort** |
- 29* |
- -15 |
- 19 |
56* 49* |
-10 26* |
47* 49* |
EQ-5D: Anxiety & Depression |
19 14 |
00 17 |
19 22* |
41 60* |
25 16 |
43* 56* |
EQ VAS° - Health State*** |
-11 -09 |
-29 06 |
-30 -05 |
-60* -75* |
-16 -31* |
-58* -73* |
Table 7 Correlations (r x 100) between BSS scores and criterion variables of SF-12 and EQ-5D. 1st and 3rd visit: placebo and verum group. °Visual analogue scale
*Significant on the 5% level
**No variance on the 1st visit, therefore no correlations
***Is reversed, i.e. high values indicate very good health
SF-12: Upper line: Study 4 (adults: n = 405)
Lower line: Study 5 (adults: n = 124)
EQ-5D: Upper line: Study 1 (juveniles aged 17 and 18 years: n = 17)
Lower line: Study 2 (juveniles aged 17 and 18 years: n = 74)
Predictive validity
Even without effective medication, the BSS total score and BSS sub scores are expected to decrease in the days following the 1st visit: It is generally accepted, that AB is a self-limiting disease. In all the placebo-controlled studies on effects of medications, the BSS total score under placebo decreased significantly from the first via the second to the third visit. In the following studies, the observation period comprised eight days.14,17,19–24 In another study,26 the period was 7-9days. Of course, longer studies likewise confirmed the assumption: 11days.27–28
SF-12: Upper line: Study 4 (adults: n=405)
Lower line: Study 5 (adults: n=124)
EQ-5D: Upper line: Study 1 (juveniles aged 17 and 18years: n=17)
Lower line: Study 2 (juveniles aged 17 and 18years: n=74)
In a double-blind setting, the BSS total scores and the BSS sub scores decrease more rapidly under treatment with an active medication than under placebo treatment: Most studies in this field have been carried out with Pelargonium sidoides (EPs 7630).13–17,19–25,34 They all affirm the hypothesis. Those studies investigating other preparations than EPs 7630 in which the BSS had been applied yielded similar results.26–28
For the clinical measurement of the severity of AB, a validated assessment scale for use in the general population is needed. Because of the usage of the BSS in more than a dozen published studies, it is an easy step to examine whether this observer rated assessment scale is valid according to the demands of classical validity theory.12,33 The presented analysis shows that the BSS can fill up the gap of a validated measure.
A high content validity of the BSS can be assumed because there is a broad consensus among recognized experts that the five symptoms of the BSS rank among the principal symptoms of AB. Similar symptoms were collected in further randomized controlled trials with acute RTI.2,41 The evidence of expertise emerges from publications controlled by peer-reviewers and Science Community. The content validity will remain stable during practical application of the BSS because the users (usually family physicians) are familiar with the range of specific symptoms and findings, given in the scale, without significant distortions.
A total of 40 principal component analyses indicate that the supposition of the construct one single factor of severity (so called “general factor”) is justifiable because the first extracted factor covers much more common variance than the second one (Table 2). The construct validity, however, increases about 68.5% when a second independent factor is adopted. In 28 of the 40 component analyses, two group factors result (Table 3) that are centred on “cough” and “sputum”.
The deviations from two group factors may be due to methodological artefacts: Over time, there are more and more patients that are assessed as healthy and are therefore without symptoms: At 3rd visit, 17.3% of the verum patients and 7.1% of the placebo patients. Severity in all items is thus practically zero. Compared to the patients who are not yet free of symptoms, there is a tendency for the correlations between the items to increase.
On the 1st visit, however, the relationships will be much less clear due to a reduced scatter of BSS scores compared to those of the next visits. For instance, the standard deviation of the BSS total score increases from 1.96 (1st visit: placebo + verum) to 2.07 (placebo) resp. 2.44 (verum) on the next visit to final 2.13 (placebo) resp. 3.03 (verum). Concomitantly, there is a tendency that the inter correlations decrease and the number of factors increases.
Without the discussed artefacts all the 40 principal component analyses might always have extracted two group factors.
The concurrent validity has been examined by reference to various criteria. In four studies, the doctors and patients evaluated the same symptoms of AB (i.e. cough, sputum production and chest pain on coughing). The patient did it at home by means of filling in the diary and the physician assessed the patient during the visit. Correlations of the same symptoms evaluated by both the physician and the patient reached a much higher level than the between BSS symptoms correlations. To get an impression, we compared the linearly interpolated median of the medians of the correlations (rx100) in the four studies: Visit 1: 77 (same symptoms) vs. 6.5; visit 2: 69.5 vs. 28; visit 3: 86 vs. 30.75. The true relations are better approached by the determination coefficients r²( x 100): Visit 1: 59.3/4.2=14.1; visit 2: 48.3/7.8=6.2; visit 3: 74.0/9.5=7.8. The value 1 would indicate “no agreement” in the judgment of the same symptom of AB. In contrast, with reference to the three selected symptoms of the BSS, the actual agreements between doctor and patient are very close. There is no indication for the two other symptoms (rales on auscultation), dyspnoea to be used much more differently between doctor and patient.
Another aspect of concurrent validity is examined by the comparison of the physician’s assessment by means of BSS and the patient’s self-evaluation of the health and current state of feeling and daily activities.
The expectation that the severity of AB diminishes is confirmed by substantial correlations with changes in subjective general health, vitality, mobility, self-care and usual activities, pain/discomfort and anxiety/depression (Table 7). These items are mainly related to the coughing complex and less to sputum. Since the BSS total score encompasses four items of the coughing complex and only one item of the sputum complex, it essentially represents the relations between the subjective health, feeling and daily activities.
Possibly, the limitation in daily activities (self-care and usual activities) accompanies AB earlier than diminishment of vitality and increase of pain/discomfort and anxiety/depression. Although AB objectively reduces health status from its start, the restriction of health seems to be subjectively hardly perceived by the suffering patient in the period when he or she initially visits the doctor.,/
Independent from the precise time course of the subjective consideration, the BSS total score and BSS subscore “coughing complex” respond to limitations that are related to AB. Thus, a further aspect of concurrent validity has been proven for these two scores at least. The concurrent validity of “sputum”, however, has less been supported in this validity aspect.
Finally, the predictive validity has been investigated. Several longitudinal studies, lasting between seven and 11 days, are published in which the BSS was administered several times under placebo conditions. According to the expectations,42 the BSS total score of each of the studies decreased during the time course.14,17,19–24,26–28
Under the influence of an active medication treatment, the BSS total score decreased more rapidly.13–17,19–28,34 These evidences for the predictive validity are also true for the BSS sub score “coughing complex” but only partially for the BSS sub score “sputum” (Figure 1), since the latter does not decrease under placebo within the 8 days from the 1st visit on. The particular course of sputum can be seen under the viewpoint of an additional evidence of concurrent validity because it refers to a complementary criterion, namely a second independent factor of AB that undoubtedly belongs to this syndrome as the discussion of content validity showed.
Generalizability of BSS administration
For the practical application of the BSS, it is important to mention that in the studies cited in Table 1 the assessment with the BSS appears to be largely independent from the specialist training of the assessing general practitioners, of their nationality and mother tongue or their age and sex. The same applies to the patients being assessed: their age and sex does not appear to have a systematic effect on the BSS findings.
The BSS is a valid measurement instrument for assessing the severity of AB. This holds true for the use of the total score and even more so for the subdivision into the sub scores “coughing complex” and “sputum”. The two factors have also been emphasized in a meta-analysis by the Cochrane Collaboration.34
If standardization of the interpretation was to be made, the BSS could be described as a “Test” according to the rules of psychometrics.12 In this way it would stand out from “mere” measurement instruments. There might result a one-dimensional and a two-dimensional test “BSS”. It should be taken into account that the latter is closer to the reality and therefore even more valid than the former.
Both components can be presented using the same framework of severity, for examples by means of graphs (Figure 1). These could be used not only for testing in a differential manner the success of interventional measures in patient groups but also for recording the course of illness in individual patients with AB.
None.
The author declares no conflict of interest.

©2014 Lehrl, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Tuberculosis Day (March 24) provides the opportunity to raise awareness about TB-related
problems and solutions and to support worldwide TB-control efforts. While great strides have been made to control and cure TB, people
still get sick and die from this disease in our country. On this event, we request researchers to spread more information and awareness
on this by their article submissions towards our JLPRR. For this we are rendering 25% partial waiver for articles submitted on or before
March 24th.
World Tuberculosis Day (March 24) provides the opportunity to raise awareness about TB-related
problems and solutions and to support worldwide TB-control efforts. While great strides have been made to control and cure TB, people
still get sick and die from this disease in our country. On this event, we request researchers to spread more information and awareness
on this by their article submissions towards our JLPRR. For this we are rendering 25% partial waiver for articles submitted on or before
March 24th.