Journal of
eISSN: 2373-4469


Mini Review Volume 3 Issue 3
1Department of Zoology, New Alipore College, University of Calcutta, India
2Department of Zoology, Bangabasi Morning College, University of Calcutta, India
3Department of General Surgery, West Bengal University of Health Sciences, India
4Human Genetics Unit, Indian Statistical Institute, India
Correspondence: Nilabja Sikdar, Ramaligaswami Fellow,Human Genetics Unit, Indian Statistical Institute, 203, BT Road,Kolkata-700108, West Bengal, India, Tel +91 9830780397, Fax +91 33 25773049
Received: August 31, 2016 | Published: November 18, 2016
Citation: Dey S, Chatterjee S, Ghosh S, et al. The geographical, ethnic variations and risk factors of gallbladder carcinoma: a worldwide view. J Investig Genomics. 2016;3(3):49-54. DOI: 10.15406/jig.2016.03.00051
Gallbladder carcinoma (GBC) is the most common malignant tumor of the biliary tract throughout the world with marked ethnic and geographical variations. GBC is the highly fatal disease with poor prognosis. GBC appears to develop dysplastic mucosa that progress to carcinoma in situ and then to invasive carcinoma. The incidence of GBC varies geographically with higher rates in certain areas of Latin America (Columbia, Peru and Ecuador), North America (Hispanic and American Indian populations) Eastern Europe (Poland, the Czech Republic, Slovakia, Hungary, and the former East Germany) and Japan. Mapuche Indians of Chile show the highest incidence and mortality of GBC. In India, GBC is more common in women in the North, North-East and East compared to the Southern part, The incidence of GBC is very high in the northern cities like Uttar Pradesh, Bihar, Orissa, West Bengal and Assam (e.g. in Delhi it is 4.5 per 100,000 for men and 10.1 per 100,000 for women) compared with the southern cities (in Chennai, Mumbai, Trivandrum, and Bangalore) incidence is 1.2 per 100,000 for men and 0.9 per 100,000 in women. Cholelithiasis/gallstones are found in 65-90% of patients with GBC. Apart from gallstones and gender biasness, a number of other risk factors favor the development of GBC like obesity, reproductive factors, genetic susceptibility, ethnicity, chronic infections and inflammations and environmental exposures to specific chemicals. Elucidating such risk factors not only provides insight into its pathogenesis accounting for its geographic and ethnic variances, but more importantly should yield strategies to prevent and treat this unusual malignancy.
Keywords: Gallbladder carcinoma; Geographical variations; Ethical variations; Cholelithiasis; Chronic inflammations; Gallbladder polyps; Porcelain gallbladder
GBC: Gallbladder Carcinoma; APBDJ: Anomalous Pancreaticobiliary Duct Junction; PBM: Pancreaticobiliary Maljunction; HCPBD: High Confluence of Pancreatobiliary Ducts; APOB: Apolipoprotein B; ABC: Adenosine Triphosphate-Binding Cassette
Gallbladder Cancer (GBC) was first reported in 1777 and most GBC carcinomas (60%) originate in the fundus of the gallbladder, 30% in the body, and 10% in the neck and over 90% are adenocarcinomas, originating in the glandular epithelium [1,2]. GBC usually results in asymmetric thickening of gallbladder wall and infiltration of surrounding structures. On gross examination, approximately 10-37% of GBCs cannot be identified with certainty, and their macroscopic appearances are similar to those of tissues affected by chronic cholecystitis. GBC is an uncommon but highly malignant tumor. Females are two to six times more affected than males and usually develop this cancer in their late 50s. In Chile GBC is the highest incidence, and second cause of death by malignant tumor in women 5:1 compared with men (http://globocan.iarc.fr/Default.aspx.2012). GBC ranks 20th among all malignant tumors, with an incidence of 2.2 x 106 inhabitants 22nd for mortality (0.7 x 106) and prevalence at 5 years of 16.5 x 106 inhabitants (http://globocan.iarc.fr/default.aspx.2012) (Figures 1&2) [3]. According to recent data published by Indian Council of Medical Research, the highest incidence worldwide to the tune of in India, there is about 800,000 new cases of GBC and 550,000 deaths per year. GBC is the most common abdominal malignancy in India [4]. With respect to other parts of the world, Northern India (Uttar Pradesh, Bihar, Orissa, West Bengal, and Assam) is a very high risk zone of GBC [4-7]. Both environmental and genetic factors are acting on GBC development but the understanding of the causes of GBC is still incomplete. However, the wide geographical, ethnic, and cultural variations in the risk of GBC suggesting there are extensive overlaps between genetic and environmental factors in the progression of the disease. Adenocarcinoma is the most common histologic type, accounting for 98% of all gallbladder tumors, two third of which are moderately differentiated, the remaining common histopathological variants include papillary, mucinous, squamous and adenosquamous subtypes. Other rare types of gallbladder malignancies include carcinosarcoma, small cell carcinoma, lymphoma, signet ring cell type tumors and metastases [8]. GBC can be subdivided into infiltrative, nodular, combined nodular infiltrative, papillary, and combined papillary infiltrative forms. Tumors may contain more than one histological variant. GBC is often diagnosed late when the disease is at advanced stages mainly due to its anatomical location, nonspecific and vague symptoms. It is difficult to diagnose clinically. In case of GBC direct invasion of the tumor into adjacent liver segments IV and V and other surrounding organs such as the duodenum, colon, anterior abdominal wall, and common hepatic ducts, is the most common mode of spread. Symptoms of GBC includes pain in the right hypochondrium, weight loss, anorexia, nausia and vomiting, lump in the right hypochondrium, jaundice, abdominal distention, pruitis, haematemesis, malena [9]. Surgery is the only curative therapy for GBC. However, at diagnosis, less than 20% of patients are candidates for curative surgery. In India however, the majority of GBC are reported at an advance stage during ultrasonography for upper abdominal symptoms. In case of metastatic GBC Median overall survival in best supportive care and 5 FU/leucovorin groups was 4.5 and 4.6 months respectively, versus 9.5 months in gemcitabine plus oxaliplatin group [8]. Metaplasia in the gallbladder wall could develop either into dysplasia which could play significant role in gallbladder carcinogenesis or it may develop into atypical hyperplasia and from there on to in situ and finally invasive cancer. In this review we analyze the worldwide geographical distribution of GBC [8]. Here we emphasized on the incidence and prevalence of GBC in Indian subcontinent. We also provide comments of several risk factors including cholelithiasis, ethnicity, infection, inflammation, gallbladder polyps, and environmental and occupational exposures, diet, lifestyle and genetic factors associated with the disease.
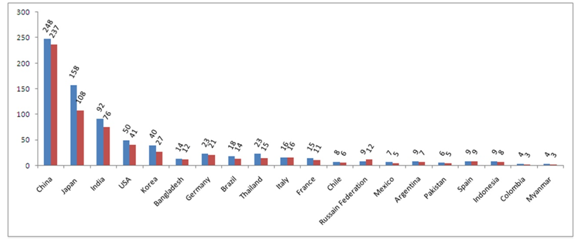
Figure 1: World's most GBC prevalent countries and rate of incidence for last 5-years (till 2012) for male population.
Last five years record of GBC in males in different countries. Steel blue bars denote estimated value (×100) of 5-year Prevalence; Crimson bars denote estimated value (×100) of Incidence. X-axis represented Worldwide distributed different countries. Y-axis represented estimated values/numbers (×100). Here Steel Blue colored bars denotes 5-year Prevalence of GBC and Crimson colored bars denote Incidence of GBC.
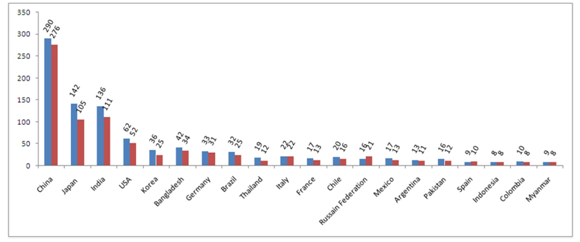
Figure 2: World's most GBC prevalent countries and rate of incidence for last 5-years (till 2012) for female population.
Last five years record of GBC in females in different countries. [Steel blue bars denote estimated value (×100) of 5-year Prevalence; Crimson bars denote estimated value (×100) of Incidence. Crimson bars denote estimated value (×100) of Incidence. X-axis represented Worldwide distributed different countries. Y-axis represented estimated values/numbers (×100). Here Steel Blue colored bars denotes 5-year Prevalence of GBC and Crimson colored bars denote Incidence of GBC.
Sex and age biasness
Women are two to six times more frequently affected by GBC than men and the occurrence steadily increases with the increasing age, high parity, early menarche, late menopause, first child birth at young age [2,10-13], perhaps it varies greatly in different parts of the world. The rates are highest in women from LA Paz, Bolivia (15.5 per 100,000). Intermediate rates (3.7 to 9.1 per 100,000) are reported from Trujillo, Peru, Quito, Ecuador, Cali, Colombia, Porto, Alegre, Brazil, Asuncion, Paraguay and Italy; in contrast in Nagasaki, Japan, among Hispanics of Central California, in Haut Rhin, France, Graubunden, Switzerland, Croatia, Shanghai in China, Montevideo, Uruguay and in the UK (England and Wales) this proportion was less reported. In North America, low rates predominate, with the exception of high rates reported among Indians in New Mexico (11.3 per 100,000) and moderate rates among female immigrants from Latin America [2,14,15]. In some countries the incidence of GBC is also very high in males; from La Paz and Bolivia 7.5 per 100,000 cases have been recorded. Similarly, from Quito, Ecuador it is 4.1 per 100,000 [2].
Geography and ethnicity
From various epidemiological data or reports it has been observed that the occurrence of GBC is somewhat location specific, so we could consider that the geographical barrier may be one of the important factors that make the frequency of GBC variable worldwide. High rates of GBC have been recorded from Chile (27 per 100,000), India (21.5 per 100,000), Poland (14 per 100,000), Japan (7 per 100,000) and Israel (5 per 100,000) [16]. From various records on GBC Northern India, Korea, Japan central/eastern Europe including Slovakia, Czech Republic, and Slovenia are also considered to be at the top of the list for occurrence of GBC [14-17]. The populations with the highest frequencies of GBC reported, are Chileans [18], Bolivians [19], North American Indians [20], Mexican Americans [21,22], and central Europeans [23,24]. Comparatively lower incidence of GBC has been recorded from the western world including USA, UK, Canada, Australia, and New Zealand (0.4-0.8 in men and 0.6-1.4 in women per 100,000) [25]. Even the occurrence of GBC may vary within the same country. In India, for example, the incidence of GBC is very high in the northern cities like Uttar Pradesh, Bihar, Orissa, West Bengal and Assam (e.g. in Delhi it is 4.5 per 100,000 for men and 10.1 per 100,000 for women) compared with the southern cities (in Chennai, Mumbai, Trivandrum, and Bangalore incidence is 1.2 per 100,000 for men and 0.9 per 100,000 in women [9,15,26]. In Lucknow (capital of Utter Pradesh), every year approximately 50-60 patients with GBC are operated and large number of GBC patients are remain un-operated due to poor physical condition and/or advanced stage of GBC [6]. In the Northern subcontinents of India the occurrence of GBC is also very high, including Pakistan (13.8 per 100,000) [14,27]; Bangladesh (67 out of 154 pancreaticobiliary cancer patients were observed with GBC) [6]. Some reports also suggest that occurrence of GBC widely varies based on different ethnicity and races. It was observed that in United States white people of both males and females are being affected 50% more frequently than the black people [9,21]. Mapuche Indians in Chile showed the highest incidence and mortality rate of GBC (12.5 x 106 in men and 27.5 x 106 in women) however, in Indians of New Mexico with an annual average rate of 9.0 x 106. Gallstone is a major important risk factor for GBC. Roa et al. [3] described among 90% of GBC have been associated with gallstones [3]. Highest incidence was observed in the indigenous populations of America and Indians. Pima Indians in the America, Mapuche women of Chile, and Eastern India has been reported with the prevalence of 75, 50, and 21.5% of gallstone respectively [2,23,28,29].
Obesity, diabetes, and dietary factors
The wide variation in occurrence of GBC may be due to variability in food habit in different regions. Although there are no sufficient data available to link GBC with dietary habit/nutritional condition but we know that excessive consumption of spicy/oily foods with calorific value, carbohydrate, red meats, red chili peppers, and oil may results in obesity; whereas green leafy vegetables and fruits may be body protective [10,30]. The obesity is one of the risk factors for development of GBC. Obesity with Body Mass Index (BMI, kg/m2) more than 30 kg/m2 increases the risk for development of various types of cancer, including GBC [10,31]. For each 5-point increase in BMI, the relative risk for developing GBC increases 1.59 times for women and 1.09 for men [29,32-34]. Diabetes mellitus and other factors related to obesity are also play important role in development of gallstones and GBC. There may a high risk for development of GBC in diabetes patients, even in the absence of gallstones [33,35].
Cholelithiasis (Gallstones)
It is the most important risk factor for development of GBC. The rate of GBC occurrence is increases 8.3 x higher in the gallstone patients than the general patients [16]. It has been observed that 65-90% of GBC patients are associated with gallstones [9,16,36]. The frequencies of gallstones and GBC run in parallel in a defined population; and the risk of developing GBC increases in direct proportion to gallstone size [37]. Patients with gallstones size >3cm having relative risk 9.2-10.1 times higher chances of GBC, greater than stones <1cm [14,38]. An autopsy data from Chile suggested that the risk of GBC is seven times greater for the patients with gallstones than the persons without gallstones [23]. Even at the presence of gallstones the risk of GBC varies widely in different ethnic groups [19]. The Age-adjusted incidence in men and women with gallstones was 16.2 and 46.4 in American Indians, as compared to 2.3 and 3.0 in American blacks, and 4.5 and 11.5 in Swedish whites, respectively [6,39]. Gallstones or biliary duct stones are hypothesized to cause chronic inflammation leading to dysplasia; but the exact mechanism by which cholelithiasis causes GBC is not clear or controversial. Chronic mucosal irritation and damage due to mechanical forces exerted by the gallstones may be involved in development of GBC [40]. Further supportive evidence comes from reports of GBC in patients who had received gallbladder preserving therapies for cholelithiasis [17], and finding of epithelial dysplasia, atypical hyperplasia, and carcinoma in-situ (seen in 83%, 13.5% and 3.5% respectively), gallbladder removed for treatment of gallstones [26].
Calcified or porcelain gallbladder and chronic inflammation
The term “porcelain gallbladder” refers to the pathological finding of a brittle gallbladder with bluish discoloration resulting from extensive calcification of the organ wall, causes chronic inflammation leads to GBC [9,29]. Less than 1% of gallbladder specimens demonstrated this change and it is frequently (approximately 25%) associated with GBC [41,42]. The premalignant lesions explain the fact, only gallbladders with partial calcification, stippled or multiple punctuate calcification in the glandular spaces of the mucosa. Due to continuous inflammations and calcifications muscularis appears. Chronic bacterial infections also cause irritations and inflammations in the gallbladder. Salmonnela typhi carriers developed GBC and risk increases 10 folds. Japanese and Thai patients of GBC having Helicobator bilis infection with odds 6.5 and 5.9 respectively [43]. Recent reports suggest that there is a much lower incidence of GBC associated with diffuse intramural calcification (type I; complete) than with selective mucosal calcification (type II and III; incomplete) [29,44].
Gallbladder polyps
Some experimental studies suggest that presence of polyps in gallbladder may increases the risk factors for development of GBC. Nearly 5% of all adults have gallbladder polyps but the majority are pseudopolyps including cholesterolosis (60% of gallbladder polyps), adenomysis (25%), or inflammatory (10%) with no neoplastic potential [29]. Potential gallbladder polyps include non-neoplastic (hyperplastic and inflammatory) and neoplastic polyps (adenomas, leiomyomas, fibromas, and lipomas). Benign adenomas, constituting 4% of all gallbladder polyps, play an unclear role in neoplastic transformation; however, the absence of adenoma remnant in mucosa adjacent to adenocarcinoma suggests these tumors may not play a role in carcinogenesis in all cases [29].
Individuals with polyps in gallbladder with diameter larger than 10 mm and age above 50 years have the greatest malignant potential [29,45]. If polyps diagnosed in asymptomatic patients, even in the absence of gallstones, removal of the gallbladder is recommended [45]. Small polyps with diameter less than 10 mm need only be removed if they are producing symptoms or are associated with gallstones [9]. A recent study suggests that polyps larger than 2 cm are more likely to harbor high grade dysplasia/malignancy and concluded to remove, whereas those <2 cm can be followed by serial ultrasound every 3-6 months [46]. Some other investigations also revealed that up to 40% of the malignant gallbladder polyps may be <1cm in size and thus patients with polyps of 5-10 mm should not be excluded from investigation/treatment [47].
Anomalous pancreaticobiliary duct junction
Anomalous pancreaticobiliary duct junction (APBDJ) or Pancreaticobiliary maljunction (PBM) is one of the risk factors of gallbladder carcinoma [10,13,28,29,48-51]. This is an abnormal union of the biliary and pancreatic ducts located outside the duodenal wall in which a sphincter is not present. Therefore, pancreatic juice can freely flow back into the gallbladder causing bile stasis, which causes to chronic inflammation and genetic alterations, leading to increase cellular proliferation resulting hyperplasia/dysplasia and then carcinoma [25,29]. Approximately 10% of gallbladder carcinoma patients have this kind of anomalies [29]. Patients those develop gallbladder carcinoma in relation with APBDJ are mostly young and have lesser incidence of gall stones [9]. Among non-APBDJ patients the back flow of pancreatic juice may occur secondary to a long common channel or high confluence of pancreatobiliary ducts (HCPBD) [8]. Approximately 38% of gallbladder carcinoma patients have been observed with more than 8 mm channel length, whereas only 3% in normal gallbladder individuals [25]. A striking report regarding APBDJ has been observed that this is prevalently occur in the patients with GBC in far Eastern countries but in the Western countries it does not seem to be significant in GBC [52,53].
Infections
Bacteria induced degradation of bile acid is a causative factors of gallbladder adenocarcinoma [16]. Helicobacter, Salmonella typhi, S. paratyphi, Ophisthorchis viverrini, Clonorchis sinensis, liver flukes, are the most common types of infection in GBC [29]. Colonization of bacteria, like chronic bacterial cholangitis, usually due to salmonella and helicobacter, increases the risk of gallbladder carcinoma [29]. GBC is more common in patients with Mirizzi’s syndrome, and typhoid carriers are a high-risk group. Salmonella typhi (~6% of carriers develop GBC: A 12-fold risk increase) and Helicobacter bilis have been implicated in GBC [19,29,45,54-59]. In an Indian study, recently reported presence of non-typhoidal Salmonella species increased the risk of GBC. A report suggested that in Indian subcontinents the rates of occurrence of GBC are very high and Salmonella infection is also considerably severe [60].
Carcinogens, environmental and occupational exposures
Carcinogens are one of the important factors causing GBC. Experimental studies have shown that some drugs including nitrosamines, methylcholanthrene, O-aminoazotoluene and isoniazide increase the risk of GBC [61]. Prolong exposure to some heavy metals may also develop GBC, including selenium, zinc, copper, lead, cadmium, chromium and nickel. Significantly lower level of selenium and zinc; and higher level of other heavy metals have been reported in serum and bile of GBC patients in comparison to the control group of patients with cholelithiasis [30,62]. Workers in oil, paper, chemical, rubber, shoe, textile, and cellulose acetate fiber manufacturing have an increased risk of developing GBC [29,30,62]. An increased risk of GBC was also reported among miners exposed to radon. In north India, the use of mustard oil loaded with carcinogenic impurities has been suggested as an aetiological factor of GBC [9]. Tobacco is also well known factor associated with GBC and use of oral contraceptive pills (containing estrogen) may also develop GBC [19]. In female secretion of excessive sex hormones enhances the secretion of cholesterol and xenobiotics into the bile. Impaired contraction of gallbladder (results from hyper secretion of progesterone and cholesterol into bile) increases residence time and it exposes the organ to some environmental carcinogens to act over there [59]. GBC patients have a high concentration of free radical oxidation products and secondary bile acids when compare with a control patients with gallstones [63-65].
Genetic factors
Beside, the environmental factors genetic factors are also very well known for development of GBC, although the details mechanism of GBC development by alteration in genetic or molecular level is still unexplainable. Individuals with a family history of GBC have high risk for development of GBC. GBC has also been associated with multiple familial polyposis/Gardner syndrome, Peutz-Jeghers syndrome. In GBC, variants of the apolipoprotein B (APOB) gene is responsible for APOB function which leads cholesterol functions in liver, has been associated with risk of GBC. Cholesterol lithogenic genes are highly prevalent in different ethnic groups, Mapuche Indians and Hispanics from Chile, and Maoris from Easter Island. Chilean Indians and Hispanics populations showed with the highest mortality rates of GBC. Studies strongly supported these population has some genetic effect for favouring the production of lithogenic bile. The Adenosine triphosphate-binding cassette (ABC) is a lipid transporter in the canalicular membrane. Polymorphisms in ABCB4, resulting defective protein synthesis leads to decreased lecithin secretion and stone formation. Genomic alterations of ABCG5/G8 results in increased cholesterol secretion into bile, making it an important susceptibility factor of cholesterol cholelithiasis, and increase risk to GBC [18,65,66].
Carcinoma of gallbladder is a highly lethal and aggressive disease with poor prognosis. The progression of disease is rapid with below 5 yrs survival. Early detection of the disease and proper treatment strategy is the key to increase the survival rate. Location specific and ethnic variation is observed in case of GBC. Geographical barriers can control the frequency of GBC worldwide. Occurrence of GBC also varies within in a country like India, where incidence of GBC is high in northern and eastern part, compared to south. Environmental influence including diet and lifestyle and genetic factors are involved in GBC progression and there is also a positive correlation between gallstone and the risk of GBC, though this association is still undefined. In India also, the environmental and comprehensive genetic factors behind GBC development from cholelithiasis is still poorly understood. The identification of the genetic factors and to analyze the environmental factors is very crucial to detect the missing link between Gallstone and GBC progression. To reveal these unknown factors, cancer genomics and new molecular biology techniques may be very helpful to resolve the mystery of GBC progression and that will also open modern therapeutic approach to increase the survival rate of GBC patients.
# S.D and S.C contributed equally to this work; S.D and S.C wrote the review, S.G did help in the clinical and pathological part of the review, N.S wrote, supervised and correspondence the review work. All authors read and approved the final manuscript. Thank to G.S and Bidyut Roy from the Human Genetics Unit, Indian Statistical Institute for critically reviewing the manuscript. N.S is supported by Ramalingaswami Re-entry fellowship (BT/RLF/Re-Entry/05/2012) from Department of Biotechnology, Govt. of India.
The authors declare that they have no competing interests.

©2016 Dey, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.