Journal of
eISSN: 2373-6453


Research Article Volume 9 Issue 1
1University of Health and Humanities, Virgin Islands, USA
2Department of veterinary medicine, Madingley road University of Cambridge, UK
3University of Science Arts and Technology, Monserrat, BWI, UK
Correspondence: AR Awan, Dean and Professor, University of Health and Humanities, Tortola. British Virgin Islands Life member Darwin college University of Cambridge UK
Received: December 10, 2021 | Published: December 23, 2021
Citation: Awan AR, Tulp OL, Field HJ. Viremia in Equine Herpes Virus-1 infection and a possible link to Transient Protective Immunity. J Hum Virol Retrovirology. 2021;9(1):11-16. DOI: 10.15406/jhvrv.2021.09.00238
Equine herpes virus (EHV-1) causes respiratory infections in equine, and results in abortion, paresis, neonatal death, and retinopathy and the virus may become latent following initial infection. Virus entry is via the respiratory route, and the virus replicates in the host in ciliated and non-ciliated epithelial cells of the respiratory tract and in Type 1 and Type 2 pneumocytes in the lung parenchyma. After viral replication in the respiratory system, the virus can become disseminated to other parts of body via viraemic cells. The virus also can cross the placenta which leads to abortion of live or dead fetuses without premonitory signs. Infected horses show transient immunity after natural or experimental infection and immune responses to EHV-1, but the immunoprotective status begins to decline after a few months of active infection. Due to the transient immune response, recovered horses are not immunoprotected and thus are prone to subsequent re-infection. Immunity is not long lived after experimental or natural infection, and as a result the development of an effective vaccine has remained a challenge.
In this study viraemic cells were studied in a murine EHV-1 infection model. Mice were infected intranasally and viraemic cells were studied on days three and five which occurs during the peak of the infection. The results of this study may help to identify the nature of viraemic cells and their role in the transient immune response to infection. Buffy coat cells and lungs were removed and stained with a fluorescent antibody test for EHV-1 antigen, and lung specimens were subjected to transmission electron microscopy. Both techniques confirmed the presence of viraemic cells in lung tissues. These viraemic cells were further stained for EHV-1 antigen, and for CD4 or CD8 biomarkers and results are discussed re: pathogenesis of EHV-1 infection, identification of viraemic cells in a murine model and possible link of viraemia to transient immune responses in EHV-1 infection, which demonstrate the validity of this murine model for the investigation of the cytopathologic mechanism and sequelae of EHV manifestation in this model.
Keywords: viraemia, cytopathic effect, CD4 lymphocyte cell expressing molecule 4/ T helper cells, CD8 lymphocyte cell expressing molecule 8, T Cytotoxic lymphocytes, CD19 lymphocyte cell expressing molecule 19 or B cells
EHV-1, equine herpes virus; FCS, fetal calf serum; CPE, cytopathic effect; ELL, equine lung cells; PBS, phosphate-buffered saline; FITC, Fluorescein isothiocyanate; DTH, delayed type of hypersensitivity
Equine herpes virus type 1 is transmitted as a respiratory infection throughout the world and is widespread among horses. This virus causes respiratory disease, and the virus crosses the placenta and causes abortion without premonitory signs, including but not limited to neonatal death, retinopathy and myeloencephalopathy leading to paresis.1–6 This virus is an alpha herpes virus and becomes latent like other alpha herpes viruses. The virus could be reactivated in its equine hosts via an unclear mechanism. Virus reactivation during periods of stress causing clinical disease and virus shedding has been reported in horses or after administration of corticosteroids.7–9 This virus causes cell-associated viraema and the viraemic cells can then spread the virus to all parts of body.10–12 The virus can cross the placenta to the foetus leading to abortion and to the CNS leading to myeloencopahapathy (paresis).13–17 This virus is so widespread and ubiquitous among horses that it is often difficult to obtain a horse free of EHV-1 infection to study the pathogenesis.9,18 A murine model was established to study pathogenesis and this model has been used in detail for vaccine efficacy and antiviral efficacy.9,19,20
Natural immunity to this virus after natural infection in horses or experimental infection in SPF foals is short lived and horses could later be re-infected with the same strain of virus.21–23A similar case has been seen in a murine model.24 In the murine model many parallels in the pathogenic responses were seen when compared to EHV-1 pathogenesis as observed in horses. Similar patterns in humoral immune responses were observed in the murine model and which started to decline after 4 weeks of infection.25 Though many equine virologists and equine immunologists have attempted to understand the mechanism of this decline in humoral immunity there still there are so many unanswered questions and to our knowledge no one knows exactly why antibody titers started to decline after infection both in horses and in the murine model. Therefore, it appears likely that this may have more to do with EHV-1 virus and the blood cells, and which may then bring about the infection and which may disrupt the cascade of interleukin signals for antibody production. Transient immune responses may be observed in mice when infected with the live virus but were not when mice were inoculated with a heat inactivated dead virus.24
Due to transient immunity this virus is a challenge to the equine industry and vaccinologists who have been attempting to develop an effective vaccine. Due to the poor understanding of pathogenesis of transient immunity the production of an effective vaccine remains a challenge and as a result there is still no effective vaccine available.21,22,26–28
Cell-associated viraemia is also seen in a murine model and in this study, we attempted to identify which type of blood cells became viraemic and what their possible role might be in the transient immune response. Findings of this study may provide insight to many venues, and which may help to further understand the pathogenesis of EHV-1 and to answer how and why the immune responses are very transient following EHV-1 viral infection. It should be noted that to the best of our understanding of this virus, it does not cause widespread immunosuppression to other pathogens but only transient immune response to EHV-1 infection has been noted. A possible link of viremic cells to transient immune response in the murine model and probably how the same responses may be observed in the natural host is discussed.
Mice strain
Female weanling BALB/C mice were obtained from Bantin & Kingman UK. Mice were 3-4 weeks old on arrival, were maintained for one week in pre-sterilized plastic cages with pine shavings as bedding in a conventional 16/8 light cycle at room temperature [20⁰C] to acclimatize the mice to the new surroundings and to minimize the effects of transportation and environmental stressors before any regulated experimental procedures were performed.
Virus strain and tissue culture
The EHV-1 strain AB4 was a gift from Prof Neil Edington of the Royal Veterinary College, London, UK. This strain of EHV-1 was originally isolated from a case of equine herpes with neurological complication (paresis). The virus was grown in rabbit kidney fibroblast (RK-13). The RK-13 fibroblast monolayer was grown in Eagles’ minimum essential medium (EMEM) supplemented with 10% newborn fetal calf serum (FCS). Cell culture was maintained at 37⁰C in a humidified atmosphere containing 5% CO2. Virus was propagated in the RK-13 cells in EMEM supplemented with 2% FCS at a low multiplicity of infection (m.o.i.) and the working stock was stored at -70°C in small volumes until used.
Intranasal inoculation of mice with live virus
Mice were slightly anaesthetized with ether and 20 µl of virus suspension was placed in each nostril untill all was inspired, which typically occurred within a few seconds. When all mice had been inoculated, the surplus virus was titrated to confirm the dose administered.
Preparation of hyperimmune sera to detect virus antigen in mouse tissue
The EHV-1 virus was grown in Equine lung cells (ELL) in large roller bottles (850cm2). When the cytopathic effect (CPE) reached 100%, the confluent of the infected cells was harvested and following centrifugation cells were resuspended in 8ml EMEM. The harvested material was then sonicated, and the virus inactivated using 0.015% formalin. This preparation was inoculated into two rabbits with Freund’s complete adjuvant. At 2 weeks interval thereafter, further inoculations were performed with Freund′s incomplete adjuvant giving a total of 4 injections per rabbit. The second rabbit was given two additional doses of injection with live virus in Freund′s incomplete adjuvant. The rabbits were bled before inoculation and after each antigen boost and their sera tested for antibody titer. The maximum titers obtained against EHV-1 were 1/300, 1/512 (neutralizing and 1/100,000 and 1/140,000 ELISA in the first and second rabbit respectively.
Histology
Mice were euthanized by pentobarbitone sodium [50mg/ml, 0.3 ml ip] and small sections of lung tissue were removed and immediately fixed in 10 % formal saline. Tissues were paraffin-embedded, and thin sections prepared using standard established methods.
Clinical assessment
Mice were examined daily and weighed individually. Obvious signs such as ruffled fur, crouching in corners, dyspnea, any abdominal breathing and dragging movements or deaths were noted and recorded.
Transmission electron microscopy
Tissues were fixed in 4% glutaraldehyde and stained with 4% osmium tetraoxide (OsO4] using standard techniques described elsewhere.25 After embedding and sectioning, the specimens were examined by transmission election microscopy using a Hitachi H600 microscope.
Infectious centre assays from buffy coat
Heparinized blood (containing 2 mg/ml EDTA) was collected, and leucocytes were counted. The blood was centrifuged in microfuge tubes and buffy coat was mixed win distilled water for 1 minute to lyse the erythrocytes. The osmotic balance was restored with phosphate-buffered saline (PBS) at 10-fold normal strengths. Cells were counted in a heaemocytometer, and a precise number of leucocytes were then added onto preformed monolayers of RK-13 cells. After 30 min of incubation medium [EMEM] was added and the incubation continued for a further 5 days at which time the development of plaques was determined. The cell preparations from the buffy coat were centriguged, the pellet placed on a glass slide, acetone-fixed and prepared for immunofluorescence staining and cytoflourescent examination as described previously.25,29
Immunofluorescence staining and double labelling
Buffy coat samples were subjected to indirect immunofluorescence staining using rabbit hyperimmune sera described above and goat antirabbit sera conjugated with isothiocyanate (FITC). Briefly the samples were treated for 40 minutes with EHV-1 rabbit antibody and then washed with cold saline and treated with goat antirabbit sera conjugated antibody Fluorescein isothiocyanate (FITC) and subjected to further incubation for an additional 40 minutes at 37oC in a humidified chamber, cells were washed, and results were read using an ultraviolet (UV) microscope as reported earlier.24 Conjugated antibodies against CD19 and CD20 receptor molecules could not be obtained at the time. To detect CD4 and CD8 receptor molecules of lymphocyte cells showing positive EHV-1, direct FAT technique using Rhodamine conjugated rabbit anti-mouse CD4 and CD8 antibodies were used (Invitrogen).
Clinical signs after intranasal inoculation
All infected mice began to manifest clinical signs of infection 48 hours after inoculation and by day 3 all were hunched with ruffled fur. In addition, the infected animals apparently appeared smaller and began losing weigh which was confirmed by measuring the body weights daily. The infected mice showed continuous weight reduction for 4 to 5 days after which time they gradually started to recover. From the third day irregularity in breathing was noted which became worsened by day 5. About half of the animal recovered from the infection. Mice were also placebo infected with RK-13 cells lysate only or with heat inactivated virus. None of these group showed any loss in weight or any other untoward signs of reaction to RK-13 monolayer cells.
Virus isolation
The virus was isolated from respiratory tissues (turbinate bones, trachea, and lungs). Occasional virus was also isolated from CNS, liver, and eye specimens from moribund mice. Virus was also isolated from the buffy coat cells of infected mice on day 3 and 5 of the infection.
Histopathological findings
Histopathological evidence of EHV-1 virus replication was observed by light microscopy and this finding was further strengthened by scanning and transmission electron microscopy. Virus replication was observed in epithelial lining of trachea bronchi and primary, secondary, and tertiary bronchioles and in terminal bronchioles as well as in both Type I and Type II pneumocytes in lung parenchyma preparations.
Viraemia
An important feature of EHV-1 is to establish viraemia which takes the virus to different parts of body including the gravid uterus leading to abortion and to the CNS leading to myeloencephalopathy in the natural host and this scenario was also observed in the murine model. Blood samples were processed for the presence of virus by plating buffy coat cells on a confluent monolayer of RK-13 cells to detect evidence of infectious virus. The prevalence of infectious centres or plaques appeared to be approximately 1 in 5 X 104 cells.
Immunofluorescence staining FITC and rhodamine conjugated staining
The buffy coat layers were centrifuged at low speed of 600 rpm for 10 minutes and the harvested sediment was treated with indirect FAT for virus antigen by using rabbit hyperimmune sera raised in house and goat antirabbit Fluorescein isothiocyanate conjugated antibody obtained commercially (Sigma-Aldrich). Bronchiolar tissue showed a strong signal on bronchiolar epithelial cells (Figure 1). A small proportion of cells were observed to contain viral antigen as it was not possible to see the entire cellular harvest on the slide in a single frame (Figure 2). These slides were further treated for rhodamine conjugated CD4 or CD8 molecule for T cell repertoire, but no signal was thereby indicating that these infected white blood cells were not from T cell lineage.
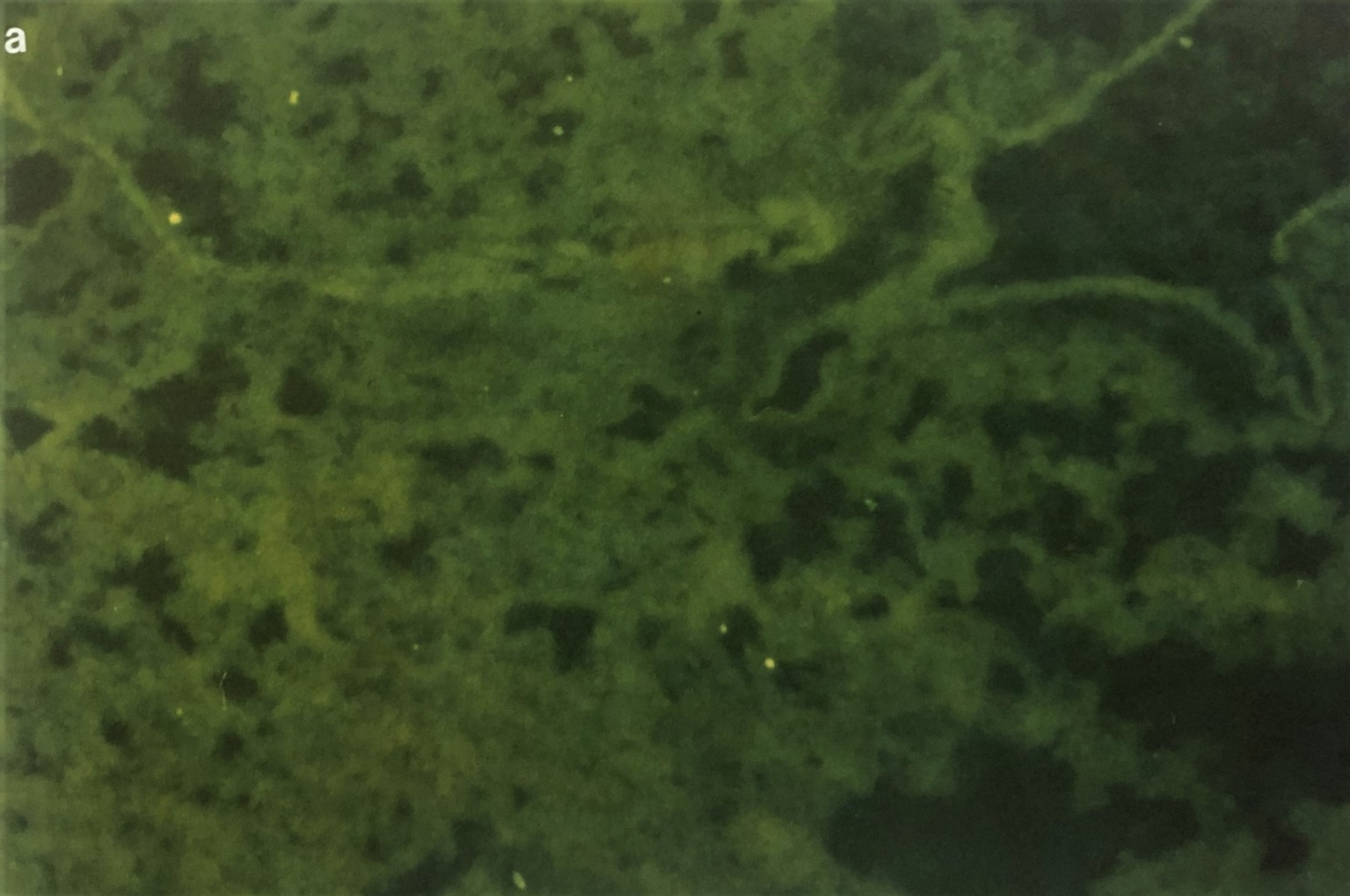
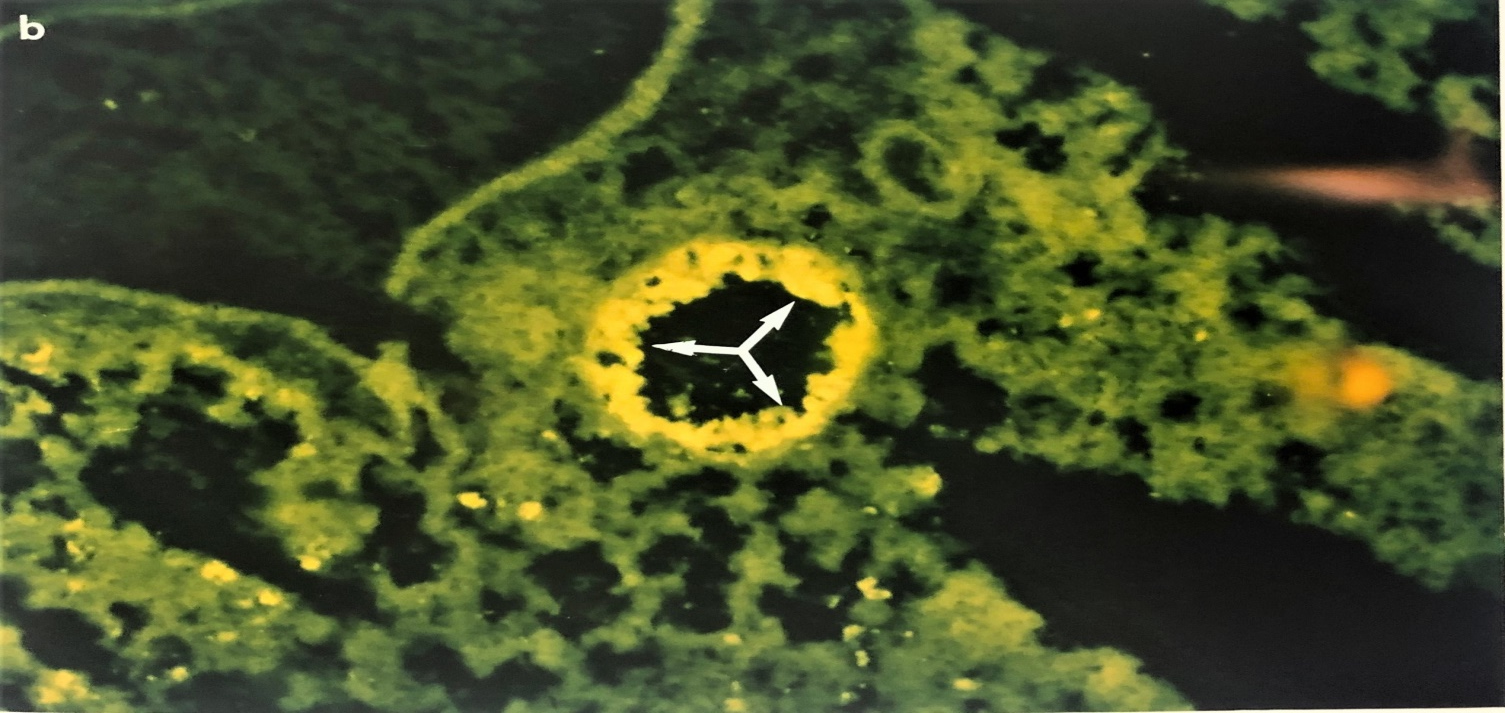
Figure 1 Photographs showing the immunofluorescent staining of EHV-1- infected mouse lung on day 3 post infection. Figure (1A) shows IF staining of EHV-1 infected lung treated with pre-immune sera. Fig (1b) showing the bronchioles on day 3 post-infected and treated with IF using rabbit anti-EHV-1 hyperimmune sera. Note that EHV-1 antigen containing antigen cells are localized in epithelial mucosa tissues (white arrow (X 193). The same techniques were used on buffy coat cells.
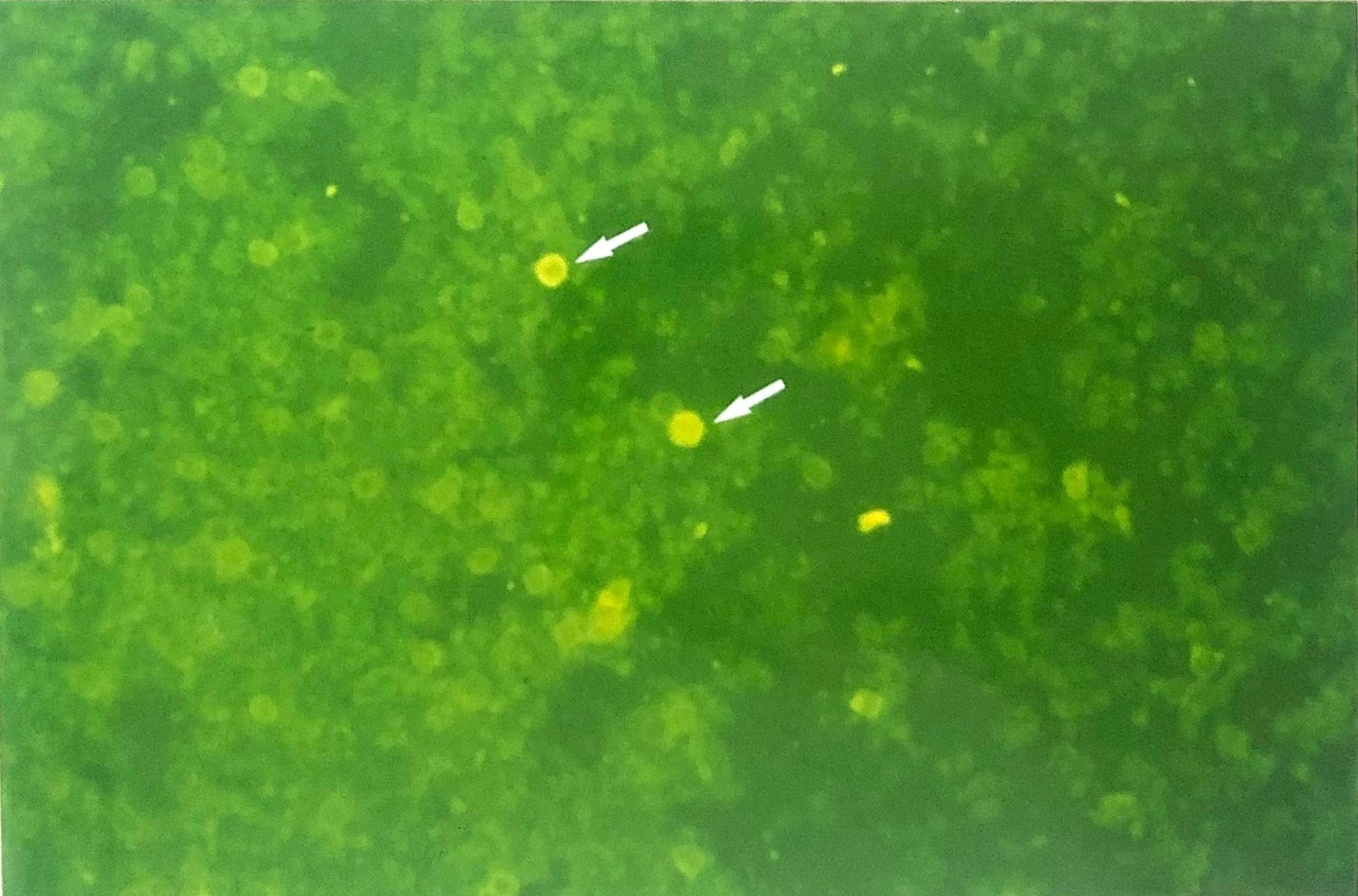
Figure 2 Micrograph showing the viraemia detected by means of immunofluorescence (indirect FAT, FITC) staining of cytospun preparation of buffy coat cells from a mouse 5-day post infection. IF staining reveals two cells containing EHV-1 antigens (X230).
Electron microscopy
The pathological changes in the lungs were more apparent in the ultra-thin sections seen by transmission electron microscopy [TEM]. In all epithelial and parenchymal cells virus replication was observed in the nucleus and typical stages of virus replication was seen including the presence of dense core virion in the nucleus and enveloped virus outside of the cells. The cell nucleus showed typical herpesvirus margination of chromatic pathology. An extraordinary observation was made when a microtome cut a section cutting right through a white blood cell and erythrocyte in a capillary. Viral core (nucleocapsid) is seen in the nucleus without an envelope which indicated the stage of virus replication in the white blood cell (Figure 3).
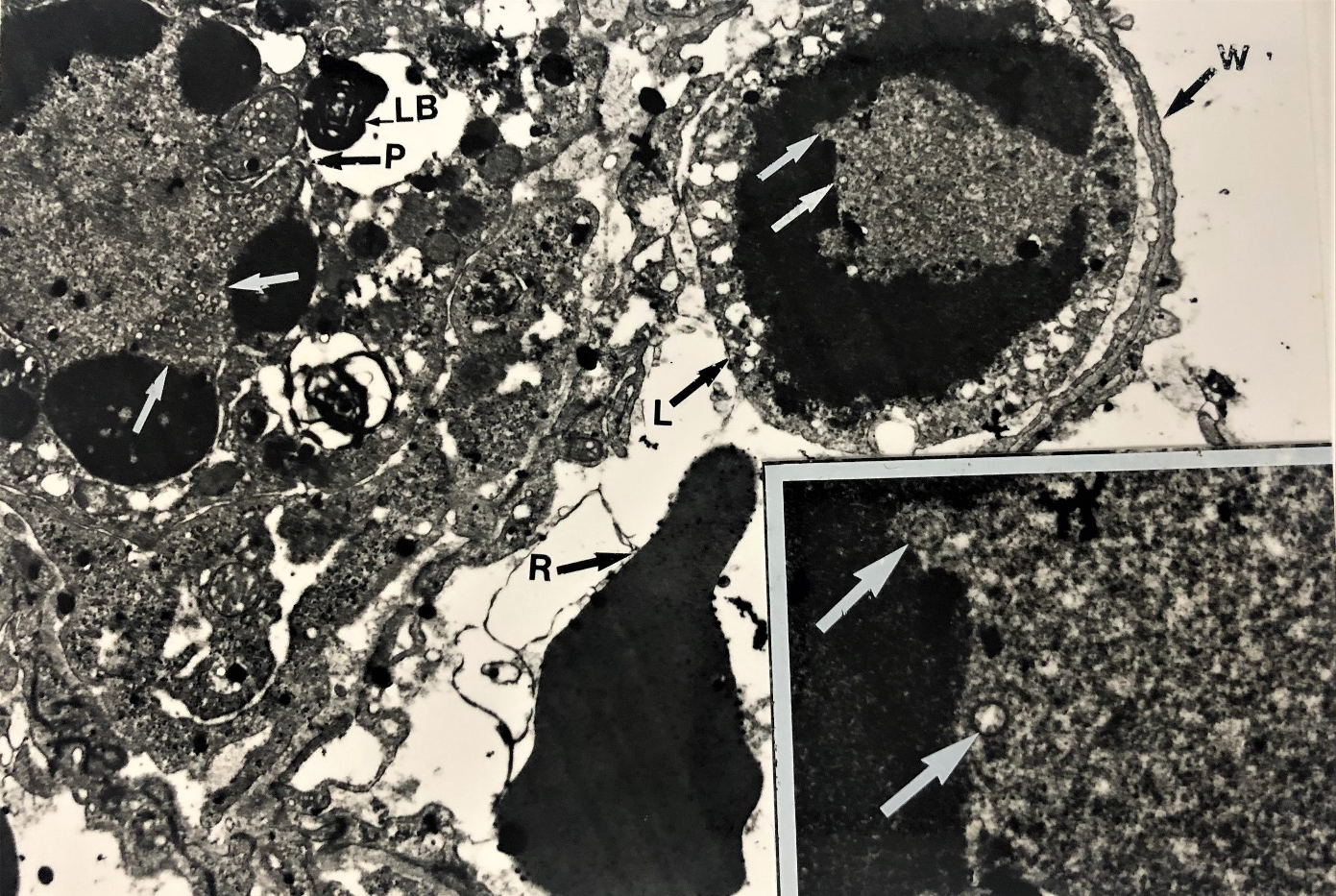
Figure 3 Electron micrograph showing evidence for productive virus replication in a leucocyte. A leucocyte contained in juxtaposition to a small pulmonary capillary adjacent to pneumocyte type 2 identified by means of lamellar bodies (LB) with the alveolar septum. The presence of nucleocapsid (white arrow) in the leucocytes (L) near the erythrocytes ® in the blood capillary wall (W). It provides a single conclusive evidence of virus replication in the leucocyte (core /nucleocapsid (X 12K). Several nucelocapsids are visible in the nucleus of this leucocyte (insert x 48 K).
After intranasal infection of mice with EHV-1 virus an active immune response both (humoral and cell mediated immune) was triggered in the murine host. However, it was noted that humoral antibody titre determined by ELISA started to wane markedly after four weeks of infection.24 This contrasts with infection of BALB /C mice with HSV where the antibody level remained high for many months.30–32 It appears that this was more likely the result of the EHV-1 and not the BALB/c mice as a transient immune response against EHV-1 is commonly found in the natural host.18,23 Plasma cells (mature B cells) contribute a major role in production of antibody against any pathogen and EHV-1 is no exception. However there appears to be a complex pathogenesis of EHV-1 as after initial production of antibody, the immune response began to decline in the murine model also24 and these mice could then be re-infected after a primary infection (Awan et al, manuscript in preparation) as opposed to HSV-1 where mice are refractory to reinfection after a primary infection.
A relatively short-lived transient humoral immune response to EHV-1 is found in horses and has been documented by many investigators and also in our lab.1,29 In addition, neutralizing antibodies were not readily detected at early time points after the primary infection of specific pathogen occurred in EHV-1 free foals.9,18 In the murine model of the present study, on the basis of single time point antibody level detected by ELISA, antibody titers fell sharply in the group of mice given the live virus. This was not observed in the inactive virus inoculated group, suggesting live virus play a possible part in the immunosuppression and transient immune response. Although no comparable or detailed information was obtained on the role of T cell responses after virus infection and as a delayed type of hypersensitivity (DTH) response could only be carried out once. No data could be obtained on the intensity of DTH with time but when the DTH response was carried out on day 30 a vigorous reaction was demonstrated. When a DTH response was carried out on the other group of mice after 78 days of EHV-1 infection it was found to still be very active, strong, and undiminished in nature.24
We found infectious virus from buffy coat cells, and this could be either cell- associated virus (attached or inside the cells) as it is from washed buffy coat cells. About 20 to 300 infectious centers were obtained per million cells on day 5 of infection.24 After staining FITC antibody for EHV-1 antigen occasional cells showed a very strong positive signal that indicated that the virus may be inside the cell perhaps replicating or merely attached to the surface of the cell as it was not possible to characterize location of virus by immunostaining when the whole cells which were demonstrating a very strong signal (Figure 1). Similarly, other herpesvirus EHV-1 also replicate in the nucleus and cause margination of chromatin material.24,33,34 This phenomenon was noted in in vitro and in ciliated and non-ciliated epithelial cells lining the respiratory tract, and in type I and type II pneumocytes in lung parenchyma and in mononuclear white blood cells in vivo. Two to 30 cells per million cells carry the virus and these cells are often hard to visualize due to their low prevalence and therefore we were fortunate to find one cell carrying the virus at a replicative stage, in addition to six nucleocapsids that were also observed in these mononuclear white blood cells (Figure 2). This lucky observation came from study during electron microscopic study where the microtome knife cut the cells approximately in half [bisected] and via transmission election microscopy one can observe a cell in a capillary located adjacent to an erythrocyte which contains virus particles during the stage of virus replication (no envelope but a nucleocapsid) and which is characteristic of herpesvirus intranuclear replication (Figure 2). This confirms and provides conclusive evidence of viraemia without a doubt in a murine model. Viraemic cells can also carry the virus to the gravid uterus in a mouse model and often results in abortion.1,14,35
By looking at the morphology of cells inside the capillary it appears that it is white blood cells which could be either neutrophil, basophils, eosinophils, or lymphocytes (B cell or T cell) or monocytes. All white blood cells (neutrophil, basophil, eosinophil, and monocytes) do not fit the morphology of this white blood viraemic cell seen in the electron micrograph which has a round nucleus and very limited cytoplasm. which is more characteristic of lymphocytes whereas neutrophils, basophils, eosinophils, and monocytes are not only larger in diameter than the erythrocytes but also will have more cytoplasm than the nucleus, and the nucleus will be either segmented or indented. Figure 2 shows a cell which has small amount of cytoplasm and large nucleus relative to cytoplasm and this morphology is more closely consistent with the morphology of a lymphocyte. Platelets will be much smaller than the erythrocytes seen in the picture and not visible in the micrograph. So, this rules out any of these cell types except lymphocytes which we believe could be important in development of viraemia in EHV-1 infection.
As is well known, only cells which have a round nucleus and limited cytoplasm are typically lymphocytes and may be distributed in blood and in the organs. These lymphocytes are small (new) or medium (mature) and/or large (aged) lymphocytes who are nearing the end of their lifespan. All these cells will have a same size large nucleus when compared to other white blood cells and smaller amounts of cytoplasm. New cell nuclei are typically more chromatic than the older cells, but this cannot be identified by electron microscopy. However, we can see that the chromatic material is denser, and the nuclei are round. You may also expect some distortion due to dehydrative processing during sample preparation of samples for electron microscopy as well as in pathological cellular changes that may be observed after virus infection has occurred.
Among the lymphocyte cells which play a role in humoral immunity are B cells which get signals from CD4 helper cells. The T helper (CD4 cells) play a major role in regulation of the immune response while CD8 cells are effector/cytotoxic T cells.36
In our DTH study, it was clear that the DTH response was very strong after infection so possibly CD8 cells were still functional. We attempted to identify the cells by double labelling the cells which carry EHV-1 antigen and for CD4 and CD8 receptor molecule. This was done by indirect FAT using FITC staining by using with rabbit anti EHV-1 sera raised in house and goat antirabbit FITC conjugated sera which gives out a green colour on UV light exposure. After first staining, these slide samples were further stained by direct rabbit anti-CD4 or CD8 rhodamine conjugated anti-mouse monoclonal antibodies which give a red colour on UV exposure. None of the EHV-1 positive viraemic cells demonstrated either CD4 or CD8 signals. In the second approach we mixed three antibodies (EHV-1, CD4 and CD8) and tried to triple stain the specimens and again cells expressing EHV-1 were strongly positive but none of these cells showed any epitope for CD4 or CD8 markers. One possibility of apparent failure of CD4 or CD8 epitopes may be that when these cells were infected with EHV-1, there is a possibility of down regulation or masking of CD4/CD8 molecules. As we mentioned earlier the DTH response was very positive so CD8 cells may not be infected in EHV-1 viremia. Furthermore, no widespread immunosuppression was observed and only a transient humoral immune response (antibody response) was observed so there is a strong likelihood that infected leucocytes with EHV-1 may not be CD4 cells. Unfortunately, we could not obtain CD19 or CD20 conjugated antibodies at the time of this project and on the basis of an exclusion test, and the known role of transient immunoglobulins and the pattern of immunoglobulin decline, we believe that these infected cells are B cells, but more work is required to address this issue. We believe that this project may open the ways and venues for other research groups to discover this part of EHV-1 complex pathogenesis.
One possibility of identifying the specific type of cells which are viremic could be achieved by using flow cytometry but since only approximately 20 cells per million became infected with EHV-1 it will be a challenging task to identify the specific cells but clearly worth pursuing. Other research groups have attempted to detect virus from the buffy coat by PCR and claimed that this is from both monocytes and lymphocytes and although these were brilliant studies that have confirmed viraemia using PCR methodology, there is no way to identify the specific type of viraemic cells by PCR.10 Another possibility is to segregate the lymphocytes into B cells and T cells (both species horse and mice) and infect them *in vitro* as this might lead us one step closer to confirmation of the specific viraemic cells in a subset of white blood cells populations.
It is well established that the cells which are important in production of immunoglobulins and switching of classes from IgM to IgG are B cells. By looking at the complexity of transient immune responses perhaps no IL-2 or IL-5 are secreted from CD4 to stimulate replication and a maturation of B cells to become plasma cells and then remain as plasma cells. This seems unlikely however as CD4 cells seem functional as they secrete IL to stimulate CD8, and we have observed a strong DTH response. Though IgM is produced early in infection and class switching has occurred as IgG is produced it is likely that B cells are functional at the onset of infection, and it also appears that CD4 helper cells are also functional. It is also possible that B cells after getting infected may lack receptors to become stimulated and thus may not produce sustained IgG production and with time these infected lymphocytes may no longer be in the circulation in sufficient numbers and memory B cells may also not be stimulated. However, this is such a complex puzzle and every step led us to unexpected findings and we wish we could have studied cytokines (IL4, IL6, IL5, IL10, IL13) as well but this study established the venues for other research groups who may also be attempting to find the answers to complex EHV-1 pathogenesis and immunity. In the complexity of EHC-1 viral pathogenesis the more we know, the more we know how little we know about this complex immunologic mechanism.
In conclusion, there is no long-lasting vaccine for EHV-1 and viraemia which can provide a strong and last lasting immunologic protection in host animals. Unless we know more about the pathogenesis of this virus and its molecular mechanism of transient immunity it will likely remain a continuous challenge to make an effective EHV-1 vaccine.
This study confirms the viraemia in a mouse model by immunofluorescent staining and by transmission electron microscopy. Viraemic cells did not pick up any signal stain with double labelling for CD4 molecule nor for CD8 molecules. Monoclonal antibodies for CD19 and CD20 receptors were not available at the time. Due to the transient nature of the humoral immune response to EHV-1 infection and the likely role of B cells (plasma cells) we strongly believe that viraemic cells observed in this study were B cells. However more research will be necessary to investigate the pathogenesis of EHV-1 infection mechanisms further as the results of this study may have implications to other viral diseases which show similarities in the transient immune responses observed in our investigation.
We gratefully acknowledge that this works was sported in part by a grant from the Equine Virology Research Foundation. A.R.A was a Cambridge Commonwealth Scholar, and we also wish to acknowledge a grant from the Jowett Trust.
The authors declare no conflicts of interest.

©2021 Awan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.