Journal of
eISSN: 2373-6453


Research Article Volume 8 Issue 3
1Kenya Medical Research Institute, Kenya
2Department of Medical Microbiology, Jomo Kenyatta University of Agriculture and Technology (JKUAT), Kenya
3Graduate School, Kenya Medical Research Institute, Kenya
4Department of Viral Infections, Kanazawa University, Japan
Correspondence: Alex Kattam Maiyo, box 54840-00200 Nairobi, Kenya, Tel (254)720252028
Received: July 15, 2020 | Published: August 19, 2020
Citation: Maiyo AK, Odari EO, Kinyua J, et al. Sero-prevalence and genotypes of hepatitis c among people who inject drugs in Nairobi Kenya. J Hum Virol Retrovirolog. 2020;8(3):86-92. DOI: 10.15406/jhvrv.2020.08.00225
The World Health Organization aims at achieve global elimination and eradication of hepatitis C virus (HCV) by 2030. Illustrating the burden of the disease among people who inject drugs (PWIDs) in Kenya is essential in management of the infection. We undertook a cross-sectional study aimed at determining sero-prevalence and genotypes of HCV among PWIDs from two dropping centers in Nairobi Kenya. Random sampling technique was used to recruit participant; a self administered questionnaire was used to obtain information on clinical history and socio-demographic factors. Missing and nonresponsive information were obtained from the facility records. Up to 5mL of whole blood samples were collected from participants whose information had been obtained. Serological analysis was done to determine sero-prevalence; molecular analysis was done on sero positive samples. Positive samples on gel electrophoresis were used for sequencing. MEGA6 was used to draw the phylogenetic tree and analyze the sequences. A total of 212 PWID were successfully recruited for the study of whom 29(13.7%) tested positive for hepatitis C antibody. Majority of them were males 21(72.4%) and females were 8(27.6%). Age (P=0.001), marital status (P=0.008), duration of injecting drugs (P=0.001) and the frequency of injecting drugs (P=0.010) were found to be significantly associated with HCV infection. 27/29(93.1%) were PCR-positive and were used for genotypic identification. HCV strains detected were genotype 1, 14(51.8%); genotype 4, 4(14.8%); genotype 6, 4(14.8%); genotype 5, 2(7.4%); genotype 3 1(3.7%) and 2(7.4%) was of undefined genotype. Earlier studies by different study groups have shown genotype 1 and 4 to be predominantly found within the region, detection of genotype5, 6 and the undefined genotypes in the current study is an indication of cross infection from other regions. This may imply a negative effect in the effort to eradicate hepatitis C in the region using the genotype specific drugs.
Keywords: hepatitis c virus, prevalence, kenya, people who inject drugs, hepatitis c genotype
AIDS, acquired immunodeficiency syndrome; CI, confidence intervals; CVR, center for virus research; HCC, Hepatocellular carcinoma; HCV, hepatitis C virus; KANCO, Kenya AIDS NGOs Consortium; KEMRI, Kenya medical research institute; MEGA, Molecular Evolutionary Genetics Analysis; NGO, nongovernmental organization; PCR, polymerase chain reaction; OR, odds ratio; PWIDs, people who inject drugs; RNA, ribonucleic acid; SERU, scientific and ethical review unit; SPSS, statistical package for social science; SSC, scientific steering committee
The prevalence of Hepatitis C virus infection continues to grow among People who Inject drugs (PWID) in Kenya despite the public health interventions that have been in existence in the last 10 years. Injecting drug use is increasingly becoming a public health concern globally and it is currently estimated that between11 and 21.1 million people worldwide inject drugs.1 The PWID are a high risk population for major blood borne infections and could act as a transmission bridge between the PWID and the general population. Studies estimate that 80% of those who suffer from HCV develop chronic infection, with 3-11 % of them developing liver cirrhosis eventually leading to hepatocellular carcinoma (HCC).2,3
In sub Saharan Africa injecting drug use is on the rise, however its statistical burden has not been fully documented since injecting drug use is still socially and legally unacceptable in many African countries. Limited data available show that Kenya, Nigeria, South Africa and Tanzania are among the countries that have reported HCV cases among PWIDs,4 with the prevalence in Kenya estimated to be between 16.4% and 22%5,6 among the drug uses in the coastal region.
Although treatment for HCV has become available in the recent past for patients who are at risk of developing cirrhosis,7 the new treatment drugs are still expensive for many low resource countries, leaving such countries still dependent on the old treatment methods which are genotype dependent.8–10 With increased global travels, it is not clear whether genotypes commonly found in one region are being spread to other regions and thus empirical management of HCV infection based on regional genotypes may not be effective. Understanding circulating genotypes will therefore be key to effective treatment of HCV infection. This cross-sectional, survey study was therefore aimed at determining the prevalence of HCV in Nairobi, a capital city in Kenya and further document the circulating genotypes among those infected.
A cross sectional study was conducted and patients recruited from two PWID dropping centers, established under the umbrella of Kenya AIDS NGO consortium (KANCO), in Nairobi between 2016 and 2017. Due to the complexity of getting the study subjects, random sampling technique was used. The recruited study subjects were aged between 18 and 67 years, and must have lived in Nairobi County for at least one Month. After informed consent and ethical clearance from Scientific and Ethical Review Unit (SERU) of Kenya Medical Research Institute (KEMRI), (SERU, SSE NO. 2209) blood samples were collected from the participants and the laboratory analysis done at the centre for virus research (CVR) in KEMRI.
Data on socio-demographic, clinical and associated risk factors
A self administered questionnaire, with the help of the clinicians, was used to obtain information on age, gender, area of residence, marital status, level of education, duration of drug injecting , needle sharing, reasons that may have lead to drug use and awareness of transmissible blood born infections. Missing data and unresponsive information were obtained from the clinic/ center records.
Laboratory analysis
The Hepatitis C serology test was done using Bioelisa HCV 4.0 kit which is an immuno-enzymatic plate coated with recombinant antigens representing epitopes of HCV: Core, NS3, NS4 and NS5.11 Viral RNA was extracted using a QIAamp RNA Mini Kit12 according to the manufacturer’s instructions. A 243 base pair (bp) fragment of HCV targeting 68-311 nt was amplified by nested PCR using HotStarTaq RNA polymerase kit from QIAGEN company and HCV specific primer pair KY80 (sense; 5’-GCAGAAAGCGTCTAGCCATGGCGT-3’) and KY78 (antisense; 5’-CTCGCAAGCACCCTATCAGGCAGT-3’) nucleotides[10].A total volume of 25µL was amplified in first-round, containing QIAGEN PCR10x buffer, 0.5µL of each primer, 10 mM dNTP mix, 2.5 Taq polymerase (HotStarTaq) and 5µL of cDNA. First round PCR consisted of 95°C for 15 min; followed by 35 cycles at 95 °C for 30 seconds; 55°C for 1 minute; 72°C for 1minute and final extension at 72°C for 10 minutes. The second round PCR was done under the same conditions using 3µl of the first-round product as template and hep21b (sense; 5’-GAGTGTYGTRCAGCCTCCAGG-3’) and hep22 (antisense; 5’-GCRACCCAACRCTACTCTCG13 as the second round primers. A total of 5 µL products of the second round amplification were analyzed by gel electrophoresis on 1.5% agarose gel stained with ethidium bromide and viewed under ultraviolet light. The HCV PCR positive samples were identified by a band at position 243bp on the molecular weight maker. From the positive samples, a volume of 15 µL was sequenced using big dye terminator to determine the circulating strains.
The query sequences were aligned along with reference sequences obtained from Gen Bank by CLUSTALW multiple sequence alignment tool on BioEdit software.14 All sequences generated were edited and the phylogenetic and molecular evolutionary analyses done using MEGA6.8 The Neighbor-Joining method on the MEGA program was used to generate a phylogenetic tree.
Data management and statistical analysis
Social demographic and risk factors data was entered and cleaned using Ms Excel and transferred to statistical package for social sciences (SPSS) version 21 and STATA version 14.2. SPSS was used to analyze the frequencies and percentages for the social demographic data, the risk factors against the HCV sero-positivity through cross-tabulation. STATA was used to perform binary logistic regression to determine factors associated with HCV infections at a confident level of 95% and a precision of 5%, p value <0.05 were considered significant.
Demographic characteristics
A total of 212 PWID participants were recruited to the study (169 males and 43 females). An overall prevalence rate of 13.7% (29/212) was established for this population, with a high proportion of those positive being male (21 of 29) (Figure 1). The average age among the PWIDs was 33.60 years; (male 34.7 female 29.4 years) as shown in Table 1.
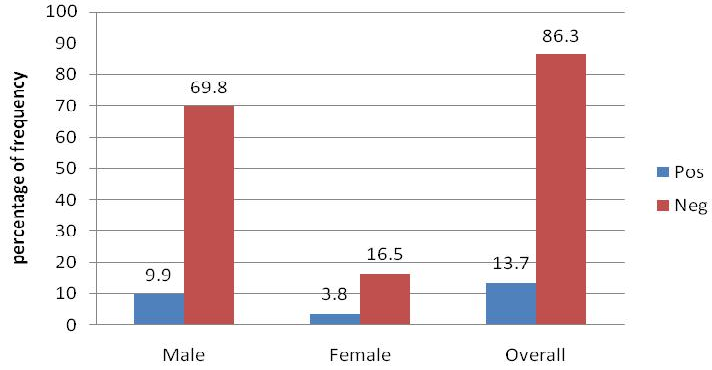
Figure 1 Prevalence of HCV among the PWIDs in Nairobi.
Showing overall prevalence of hepatitis C and the prevalence per gender group among people who inject drugs in Nairobi, Kenya (n=212).
|
Variable |
Variable group |
Frequency |
Percentages |
|
Age |
Below 20 Years |
7 |
3.3 |
|
21 Years to 39 Years |
156 |
73.6 |
|
|
40 Years to 59 Years |
43 |
20.3 |
|
|
Above 60 Years |
6 |
2.8 |
|
|
Gender |
Male |
169 |
79.7 |
|
Female |
43 |
20.3 |
|
|
Marital status |
Married |
52 |
24.5 |
|
Single |
72 |
34 |
|
|
Separated |
88 |
41.5 |
|
|
Area of residence |
Block (A) |
98 |
46.2 |
|
Block (B) |
95 |
44.8 |
|
|
Block (C) |
19 |
9 |
|
|
Gender |
Male |
169 |
79.7 |
|
Female |
43 |
20.3 |
|
|
Marital status |
Married |
52 |
24.5 |
|
Single |
72 |
34 |
|
|
Separated |
88 |
41.5 |
Table 1 Frequency distribution of PWID from the selected drop-in center in Nairobi, Kenya to different democratic factors
Within the marital status, majority (88, 41.5%) were separated or divorced, (72, 34%) were singles and (52, 24.5%) were still in marriage. Of those infected, the average age among the males was 34 years, while the female had an average of 31 years. The association between HCV sero-positivity and; age, gender and marital status variables showed varied results (Table 2).
|
Variable |
Variable group |
No./% |
% Pos within group |
% Pos within HCV |
P- Value |
Odds ratio |
95%CI for Odds |
|
Age |
below 20 Years |
0 (0.0%) |
0.00% |
0.00% |
0.001 |
6.89 |
3.049 |
|
21 Years to 39 Years |
13 (6.1%) |
8.30% |
44.80% |
15.569 |
|||
|
40 Years to 59 Years |
13 (6.1%) |
30.20% |
44.80% |
||||
|
Above 60 Years |
3 (1.4%) |
50.00% |
10.30% |
||||
|
Gender |
Male |
21 (9.9%) |
12.40% |
72.40% |
0.105 |
2.346 |
0.837 |
|
Female |
8 (3.8%) |
18.60% |
27.60% |
6.572 |
|||
|
Marital status |
Married |
11 (5.2%) |
21.20% |
37.90% |
|||
|
Single |
8 (3.8%) |
11.10% |
27.60% |
0.008 |
0.448 |
0.247 |
|
|
Separated |
10 (4.7%) |
11.40% |
34.50% |
0.812 |
|||
|
Area of residence |
Block A |
14 (6.6%) |
14.30% |
48.30% |
|||
|
Block B |
12 (5.7%) |
12.60% |
41.40% |
0.781 |
0.906 |
0.45 |
|
|
Block C |
3 (1.4%) |
15.80% |
10.30% |
1.823 |
|||
|
For how long have you been using inject-able drugs? |
< 1Year |
2 (0.9%) |
5.70% |
6.90% |
0.001 |
7.454 |
3.275 |
|
Btw 1 and 5 Years |
7 (3.3%) |
5.50% |
24.10% |
16.96 |
|||
|
over 5 Years |
20 (9.4%) |
40.80% |
69.00% |
4 |
|||
|
How often do you inject drugs? |
Daily |
26(12.3%) |
17.80% |
89.70% |
0.01 |
0.201 |
0.059 |
|
Once a week |
3 (1.4%) |
5.70% |
10.30% |
0.679 |
|||
|
Only when with my friends |
0 (0.0%) |
0.00% |
0.00% |
||||
|
How often do you share needle when injecting drugs? |
Every time I use drugs |
0 (0.0%) |
0.00% |
0.00% |
|||
|
When I cannot buy/ not given my own |
16 (7.5%) |
14.40% |
55.20% |
0.818 |
0.946 |
0.572 |
|
|
I have never shared |
1 (0.5%) |
3.30% |
3.40% |
||||
|
I cannot remember |
12 (5.7%) |
18.20% |
41.40% |
||||
|
Earlier Hepatitis test |
Yes |
3(16.7%) |
10.3 |
1.4 |
0.756 |
0.808 |
0.21 |
|
No |
26(13.4%) |
89.7 |
12.3 |
3.106 |
|||
|
Contribution to drug use |
Family member |
3(11.5%) |
10.3 |
1.4 |
0.392 |
1.345 |
0.682 |
|
My friends |
23(13.2%) |
79.3 |
10.8 |
2.65 |
|||
|
Was under stress |
3(25.0%) |
10.3 |
1.4 |
||||
|
Dangers related to shared needles |
Transmission of HIV only |
13(16.0%) |
44.8 |
6.1 |
0.815 |
1.078 |
0.572 |
|
Transmission of HIV, HBV And HCV |
11(9.6%) |
37.9 |
5.2 |
2.032 |
|||
|
I don’t know |
0 (0.0%) |
0 |
0 |
||||
|
No danger |
5(38.5%) |
17.2 |
2.4 |
Table 2 Demographic association analyzed by binary logistic regression correlation among PWID in Nairobi, Kenya (n=212)
Briefly, a significant association with age (OR 6.9; p= 0.001) was determined, where those above 60 years had a higher risk of infection compared to those below 20 years. A significant association with marital status (OR 0.4; p=0.008) was determined, where those who reported to be married were at high risk of infection compared to those who reported to be single or separated. Analysis of the association between HCV sero-positivity with duration and frequency of drug use showed varied results. A significant association with the duration of injecting drug (OR 7.5; p=0.001) was also determined, where those who had injected drugs for more than five years were at the highest risk of infection while those who had injected for less than a year being at a lower risk of infection. Frequency of injecting drugs also showed a statistical significance with HCV infection (OR 0.2 p=0.010). Those who injected drugs on a daily basis being at most risk of infection while those who injected only when with friends were at least risk of infection. The analysis of knowledge found no significance association between the following; level of education (P<0.322; OR= 1.327; CI= 0.759-2.314), having had tested earlier (P<0.756; OR= 0.808; CI= 0.210-3.106) , people who may have influence to indulging in drug use (P<0.392; OR= 1.345; CI= 0.682-2.650), knowledge of the dangers related to sharing needles (P<0.815; OR= 1.078; CI= 0.572-2.032) and HCV infection, as summarized in Table 2.
Genotypes of HCV
Of the 29 antibody positive samples, 27 samples were detected by PCR and were successfully sequenced. The most predominant genotype was genotype 1 subtype a accounting for 51.8% (14/27) followed by genotype 4 subtype a accounting for 14.8% (4/27). Four of the participants (14.8%) had the rare genotype 6 subtype a and f. two sample was classified as “undefined since they did not cluster within the known genotypes (Table 3 & Figure 2).
|
Variable |
Group |
Genotype and subtype |
||||||
|
1a |
3 |
4a |
5a |
6a |
6f |
Undefined |
||
|
Age |
21-39y |
5(18.5%) |
0.00% |
3(11.1%) |
1(3.7%) |
2(7.4)% |
2(7.4%) |
1(3.7)% |
|
40-59y |
8(29.6%) |
1(3.7%) |
1(3.7%) |
1(3.7%) |
0.00% |
0.00% |
0.00% |
|
|
Over 60 |
1(3.7% |
0.00% |
0.00% |
0.00% |
0.00% |
0.00% |
1(3.7)% |
|
|
Gender |
Male |
11(40.7%) |
1(3.7%) |
2(7.4%) |
1(3.7%) |
2(7.4%) |
2(7.4%) |
2(7.4%) |
|
Female |
3(11.1%) |
0.00% |
2(2.4%) |
1(3.7%) |
0.00% |
0.00% |
0.00% |
|
|
Area of residence |
Block A |
9(33.3%) |
1(3.7%) |
1(3.7%) |
1(3.7%) |
0.00% |
2(7.4%) |
0.00% |
|
Block B |
5(18.5%) |
0.00% |
3(11.1%) |
1(3.7%) |
0.00% |
0.00% |
1(3.7%) |
|
|
Block C |
0.00% |
0.00% |
0.00% |
0.00% |
2(7.4%) |
0.00% |
1(3.7%) |
|
|
Once/WK |
0.00% |
0.00% |
0.00% |
1(4.2%) |
0.00% |
0.00% |
0.00% |
|
|
Length of IDU |
1 Year |
1(3.7%) |
0.00% |
0.00% |
0.00% |
1(3.7%) |
0.00% |
0.00% |
|
Btw 1-5 y |
4(14.8%) |
0.00% |
2(7.4%) |
0.00% |
0.00% |
0.00% |
0.00% |
|
|
Over 5 y |
9(33.3%) |
1(3.7%) |
2(7.4%) |
2(7.4%) |
2(7.4%) |
2(7.4%) |
2(7.4)% |
|
|
Frequency of IDU |
Daily |
14(51.9%) |
1(3.7%) |
3(11.1%) |
1(3.7%) |
2(7.4%) |
1(3.7%) |
2(7.4%) |
|
Once/WK |
0.00% |
0.00% |
1(3.7)% |
1(3.7%) |
0.00% |
1(3.7%) |
0.00% |
|
|
Frequency of sharing Needles |
Can’t buy or not given |
7(25.9%) |
1(3.7%) |
3(11.1%) |
1(3.7)% |
2(7.4)% |
2(7.4%) |
1(3.7%) |
|
Have never |
0.00% |
0.00% |
1(3.7%) |
0.00% |
0.00% |
0.00% |
0.00% |
|
|
Can’t remember |
7(25.9%) |
0.00% |
0.00% |
1(3.7%) |
0.00% |
0.00% |
1(3.7%) |
|
Table 3 Association of hepatitis C genotypes and subtypes in relation to HCV risk factors among hepatitis C positive PWIDs from selected centers in Nairobi Kenya
The analysis of associations between HCV risk factors and the detected HCV genotype showed that those found with genotype 6 sharing most of HCV risk factors in common. Though the four clients were from different area of residence, they were of the same age group, all were male; all having been injecting drugs for over five years; injecting drugs on a daily basis and they shared needles whenever they cannot buy or given their own needles (Table 3).
The influence of age to the distribution of genotypes showed a relatively equal distribution of the genotypes among those in genotype 1a and 5a. However genotype 4 and 6 were more common among the age group of 20 to 39 years. The undefined genotypes were found among the age group of 20 to 39 years and above 60 years. Equally, genotype 6 was found among the age group of 21 to 39 years. In gender, genotype 1a, 3, 6a, 6f and the undefined were dominant among the male while genotype 4a and 5a were relatively equally distributed among the sexes. However the undefined were found among the males. The length of injecting drugs played a role with each of the genotypes being found among those who injected drugs for over 5 years and being the most dominant 18(66.7%) (Table 3).
We report a significant proportion of PWID population in Nairobi, Kenya infected with a diverse group of circulating HCV genotypes, among them, genotypes not previously known to circulate in Kenya. The existence of genotypes found in other regions in this population points towards a shift in the epidemiological and geographical distribution of HCV globally.
Though in our study we showed an HCV prevalent of 13.7% which is way lower compared to the global estimate of 52.3% among PWIDs, we showed relatively similar finding as a recent study by Akiyama, which showed HCV prevalence of 13% among PWIDs in Nairobi.9
A review of HCV distribution in Africa10 has shown that East and West Africa are predominantly having genotypes 1 to 3, Central Africa genotype 4, north Africa genotype1 and genotype 4, while the South Africa is dominated by genotype 5. findings which concurred with our finding which mainly saw a high rate of circulation of genotype 1a. We note however that this is not the very first study reporting the circulation of genotype 4 in Kenya, since Muasya and colleagues previously reported the circulation of genotypes 1 and 4 within a cohort of PWIDs. To the best of our knowledge however, this is the first study reporting the circulation of genotypes 5a, 6a and 6f, which is predominantly known to circulate in south Africa and south East Asia respectively.15 The continuous finding of genotype 4 and now genotype 5 and 6 (genotypes considered dominant in other regions) in the region, points towards importation of genotypes due to global migration and interactions, raising a concern on whether genotype specific treatment of HCV infections is still a noble idea. in concurrence to the study by Muasya et.al who found genotype 1a to be the most dominant in Kenya, in this study we also showed genotype 1a to be the most prevalent.6
The rate of transmission among females 18.6% was higher compared to males 12.4%. This finding could be attributed to the fact that majority 75% of the positive females, reported to be sharing needles every time they inject drugs compared to 47% of the HCV positive males who reported to be sharing needles every time they inject drugs. Similar findings were also realized in a systematic review and meta analysis of 28 studies.16 However in their analysis they could not pinpoint the higher prevalence in females to any geographical or behavioral factors. Contrary to our finding, a study done in China on general population found a higher prevalence in males than the females.17 This was postulated to be as a result of drug misuse being more common in men than women in the study.
Though participants between the ages of 21-39 years were found to indulge heavily in injecting drug use, they presented low percentage of HCV infection among the age groups. This could be attributed to the fact that majority (83.3%) of those within the age group reported to have been using injecting drugs for less than 5 years. In addition, 43.3% of the members in the age group reported to have either never to have shared needles or could not remember the last time they shared needles during drug use. Members above the age of 40 years showed an increase in prevalence of HCV. This may be due to the fact that majority of them 75.5% reported to be injecting drugs on a daily basis and 53% within the group shared needles every time they cannot buy or were not given their own needles. In concurrent with our finding, two studies found the same high prevalence among people above 40 years however no clear explanation was given in their findings.10,18 Marital status was found to be associated with HCV infection in our study, interestingly those who were married had the highest prevalence, perhaps this is as a result of majority (63.3%) of the married that were HCV positive were found to have been injecting drugs for more than 5 years; (81.9%) inject drugs on a daily basis and also (36.4%) of them shared needles when they could not buy or given their own. Although majority (54.5%) could not remember when they last shared needles; they did at least report to have shared needles at some point. These have been shown to be contributing factors to HCV transmission.19,20
The duration of drug use, the frequency of injecting drugs and the frequency of sharing drugs were found to be significantly associated with HCV infection. Majority (69%) of those who were positive had used drugs for more than five years, compared to those who had used drugs for less than five years (31%). 89.7% of the positive reported to be injecting drugs on a daily basis, and 55.2% reported to be sharing drugs when they could not buy or given their own needles. This finding concurred with early findings in a study from New York City,21 that found those who had used inject-able drugs for more than five years and a high frequency of injection to be at higher risk of HCV infection.
Our results revealed a high risk of HCV infection among PIWDs; and its association with age; marital status; duration of injecting drugs and the frequency of injecting drugs. Preventive measures and interventions should be put in place to counter these factors among the selected group and other groups that are at high risk of infection.
This study showed a possibility of shift in HCV genotypes in the region. This study witnessed incidence of genotype 5a, 6a and 6f infection in the selected group. This temporal intrusion of other genotypes has fundamental implications with regard to translational efforts aimed at eliminating and eradicating HCV in Kenya. The presence of samples that could not be assigned to a specific genotypes demands further analysis in order to develop a better understanding of HCV genotype circulating among the selected population.
Though we report intrusion of unexpected genotypes, this study did its genotypic analysis based on 5UTR region which has however been considered not sufficient for conclusive declaration of genotypes. We therefore recommend analysis of the full genome or target other regions in order to make an informed decision from the population.
The authors are grateful to the staff of the Kenya Medical Research Institute, hepatitis laboratory for their support during the study. The authors are also in debt to the Kenya AIDS NGOs Consortium (KANCO) for their support in mobilization of the PWIDs. We thank Dr. Grace Naswa Makokha of Hiroshima University, Japan for review of our manuscript.
We know of no conflict of interest associated with this publication and there has been no financial support for this study that could have influenced its outcome.

©2020 Maiyo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.