Journal of
eISSN: 2373-6453


Short Communication Volume 2 Issue 1
National Center for Bio defense and Infectious Diseases, George Mason University, USA
Correspondence: Jia Guo, Jia Guo, National Center for Biodefense and Infectious Diseases, School of System Biology, George Mason University,10900 University Boulevard, Manassas, VA 20110, USA, Tel 7039939329
Received: December 08, 2014 | Published: January 17, 2015
Citation: Liang H, Toro R, Wu Y, Guo J (2014) Effects of Tadalafil and Sildenafil on HIV Infection in vitro. J Hum Virol Retrovirol 2(1): 00030. DOI: 10.15406/jhvrv.2015.02.00030
Background: Tadalafil (Cialis, Lilly ICOS) and Sildenafil citrate (Viagra, Pfizer) are by far the most popular and widely used drugs for enhancing male erectile function. Recent studies have suggested that Tadalafil administration increases the expression of CXCR4 in human endothelial progenitor cells (EPC). CXCR4 is also a major co-receptor for HIV (Human Immunodeficiency Virus) infection of human CD4 T cells. In addition, the viral tropism switch to CXCR4 is associated with rapid disease progression in patients.
Findings: We investigated whether Tadalafil and Sildenafil increased CXCR4 expression on human CD4 T cells, which may potentially facilitate HIV infection and transmission. We found that both drugs transiently increase CXCR4 expression on CD4 T cells. We further used an HIV-1 Rev-dependent reporter cell (Rev-CEM-Luc-GFP) to evaluate possible drug enhancement of viral replication in vitro. We also found that no significant impact of viral replication was observed, when cells were transiently exposed to these drugs. In contrast, both drugs inhibited HIV-1 infection when cells were treated for extended periods of time, likely resulting from interference with intracellular signaling environment involved in HIV-1 infection.
Conclusion: These results suggest that although both drugs enhance CXCR4 expression, they may not enhance HIV replication and increase HIV transmission.
Keywords:HIV-1, Tadalafil, Sildenafil, Rev-CEM-Luc-GFP, PBMC
EPC, Endothelial Progenitor Cells; PI, Propidium Iodide; PBMC, Peripheral Blood Mononuclear Cells; DMSO, Dimethyl Sulfoxide; TMB, Tetra Methyl Benzidine
Tadalafil (Cialis, Lilly ICOS) and Sildenafil citrate (Viagra, Pfizer) are by far the most popular and widely used drugs for enhancing male erectile function. A recent study has suggested that Tadalafil administration increases the expression of CXCR4 in human endothelial progenitor cells (EPC).1 CXCR4 is also a major co-receptor for HIV infection of human CD4 T cells.2 During viral entry, the virus glycoprotein gp120 binds to CD4 and the co-receptor CXCR4/CCR5 to mediate viral fusion3 and intracellular signal transduction4 necessary for viral entry and post-entry migration in blood CD4 T cells.5-10 Clinically, the viral switch from the usage of CCR5 to CXCR4 is also associated with rapid depletion of blood CD4 T cells and disease progression towards AIDS.11-13 These well-documented roles of CXCR4 in HIV infection raise concerns regarding potential effects of Tadalafil and Sildenafil on HIV transmission and disease progress.
Indeed, it has been documented that the odds of unprotected anal sex with a partner of unknown or sero discordant HIV status increase in Sildenafil users (ranged from 2.0 to 5.7 times of non-user, mean=3.9).14 The risk of Sildenafil use and STD diagnosis among HIV-positive men who have sex with men was 1.92 (P=0.05), and the odds of Sildenafil use among those newly HIV infected was 2.5.14 Sildenafil is also increasingly used for the therapy of pulmonary arterial hypertension (PAH) in HIV infected patients.15-17 As Tadalafil and Sildenafil share the same mechanism in clinic treatment, the implications of both recreational and non recreational use of Tadalafil and Sildenafil in relation to HIV infection deserve attention.
In this article, we investigated whether Tadalafil and Sildenafil increase CXCR4 expression on human CD4 T cells, and whether this increase in CXCR4 may facilitate HIV infection and transmission. A previous study has demonstrated that Tadalafil administration increases the expression of CXCR4 expression in human endothelial progenitor cells (EPC).1 We similarly treated human Rev-CEM-Luc-GFP cells with 1 nM Tadalafil or 10 nM Sildenafil for a time course from 0 to 3 hours. Cells were then stained with a PE-Cy5 labeled anti-CXCR4 antibody, and the CXCR4 expression level was indicated by the intensity of PE-Cy5. We observed a measurable increase of CXCR4 expression after 30 minutes in Tadalafil-treated cells (Figure 1A), and the level is relatively stable during the 3 hours of treatment. Similarly, Sildenafil treatment also altered the CXCR4 density on the cell surface, and the level increased at 30 minutes after the treatment. The enhancement peaked at 2 hours, and then decreased at 3 hours after the treatment (Figure 1B).
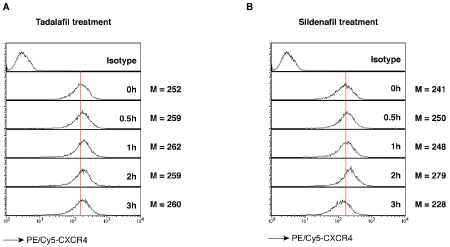
Figure 1 Surface CXCR4 receptor staining in Rev-CEM-Luc-GFP cells.
Cells were treated with 1 nM Tadalafil (A) or 10 nM Sildenafil (B) for a time course from 0 to 3 hours. Cells were stained with PE/Cy5 labeled anti-CXCR4 antibodies, then washed and analyzed with flow cytometry. The CXCR4 expression level was measured by the Mean intensity (M).
We further tested the effects of these drugs on promoting HIV infection. We used an HIV-1 Rev-dependent indicator CD4 T cell line, Rev-CEM-Luc-GFP,18,19 which expresses luciferase and GFP only after HIV infection. To measure the effects of Tadalafil and Sildenafil, we first performed transient treatment of Rev-CEM-Luc-GFP cells with these drugs. Cells were pre-treated for 1 hour with Tadalafil or Sildenafil, and then infected with HIV-1 for two hours in the presence of the drug. Following infection, cells were washed and then cultured in fresh medium for 72 hours without the drug being added back. GFP expression was measured at 72 hours by flow cytometry. Infected cells were also stained for apoptosis with propidium iodide (P.I.) during cytometry to exclude drug cytotoxicity. GFP expression will be measured only in the viable cell population (low P.I. staining). As shown in (Figure 2A), HIV-1 infection led to the GFP expression in 2.12% cells, whereas brief treatment of cells with Tadalafil in a range of dosages (from 10 nM to 10 μM) during infection did not enhance viral replication. We performed a similar infection experiment in Sildenafil-treated cells. As shown in (Figure 2B), HIV-1 infection led to the GFP expression in 1.58% cells, where as brief treatment of cells with Sildenafil in a range of dosages (from 10 nM to 10 μM) during infection did not lead to significant enhancement of viral replication (GFP+ cells, 1.89-2.20%). Similar results were observed when concentrated high titer viruses (2000ng p24) were used for infection (Figure 3).
We also investigated the drug effects on HIV infection with extended treatment of cells. As shown in (Figure 4), cells were similarly treated with the drugs as in (Figure 2), but following HIV infection, Tadalafil or Sildenafil was added back to the medium, and cells were cultured in the presence of the drug for 72 hours. Surprisingly, both drugs inhibited HIV-1 infection (Figure 4). Tadalafil slightly diminished HIV-1 replication to 44% of the control (at 10nM), where Sildenafil maximally inhibited HIV-1 to approximately 30% of the control (at 10nM). Nevertheless, both drugs did not demonstrate a clear dosage-dependency in the inhibition of HIV-1 infection. The mechanisms of inhibition by these two drugs are not clear currently, but may be related to non-specific interference with certain intracellular signaling molecules involved in HIV-1 infection.

Figure 2 HIV-1 replication in Tadalafil or Sildenafil treated Rev-CEM-Luc-GFP cells.
Cells were treated with Tadalafil (A) or Sildenafil (B) at the indicated concentration for 1 hour prior to infection with 250 ng HIV-1NL4-3 for two hours in the presence of the drug. Following infection, Cells were washed and cultured in the absence of drug for 72 hours, and then analyzed with flow cytometry. To exclude cytotoxicity, cells were also stained with propidium iodide (P.I.) and GFP expression was measured only in the viable cell population.

Figure 3 Concentrated HIV-1 replication in Tadalafil or Sildenafil treated Rev-CEM-Luc-GFP cells.
Cells were treated with Tadalafil (A) or Sildenafil (B) at the indicated concentration for 1 hour prior to infection with concentrated HIV-1NL4-3 (2500 ng) for two hours. Following infection, cells were washed and cultured for 72 hours in the continuous presence of the reagents, and analyzed with flow cytometry. To exclude cytotoxicity, cells were also stained with propidium iodide (P.I.) and GFP expression was measured only in the viable cell population.

Figure 4 HIV-1 replication in Tadalafil or Sildenafil cultured Rev-CEM-Luc-GFP cells.
Cells were treated with Tadalafil (A) or Sildenafil (B) at the indicated concentration for 1 hour prior to infection with 250 ng HIV-1NL4-3 for two hours. Following infection, cells were washed and cultured for 72 hours in the continuous presence of the reagents, and analyzed with flow cytometry. To exclude cytotoxicity, cells were also stained with propidium iodide (P.I.) and GFP expression was measured only in the viable cell population.
We also repeated the experiment in (Figure 5) using peripheral blood mononuclear cells (PBMC). As shown in (Figure 5), we did not observe significant inhibition or enhancement of HIV-1 infection by Tadalafil or Sildenafil at 10 nM. There was only a slight enhancement of HIV-1 infection by Sildenafil at lower dosages on day 11 and 13 post infection (100 pM) (Figure 5B). Our results suggest that although both Tadalafil and Sildenafil enhance CXCR4 expression on human CD4 T cells, they may only have a marginal effect on enhancing HIV replication.

Figure 5 HV-1 replication in Tadalafil or Sildenafil treated PBMCs.
PBMCs were purified from healthy donors. For HIV-1 infection, cells were pre-activated with PHA and IL-2 for 24 hours. One million cells were treated with each of the reagents for 1 hour (A for Tadalafil, B for Sildenafil), and infected with HIV-1NL4-3 (250 ng p24) for 2 hours in the presence of the reagents. Infected cells were washed and re suspended in 1 mL of fresh medium with PHA plus IL-2 and the drug added. Additional PHA plus IL-2 were added every 48 hours. Supernatant was taken for measuring viral p24 release by p24 ELISA.
Cell and Viruses
All protocols involving human subjects were reviewed and approved by the George Mason University (GMU) institutional review board. Peripheral mononuclear cells (PBMC) were purified from the peripheral blood of healthy donors from GMU using lymphocyte separation medium (Mediatech) as previously described.4 Purified cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), penicillin (50 U/mL) (Invitrogen), streptomycin (50 mg/mL) (Invitrogen), and phytohaemagglutinin (PHA) (3 µg/mL) (Sigma) plus IL-2 (100 U/mL) (Roche Applied Science). The HIV Rev-dependent reporter cell has been previously described.18,19 The Rev-CEM-Luc-GFP cell, carrying both luciferase and GFP, was cultured in RPMI 1640 complete medium. Virus stocks of HIV-1NL4-320 were prepared by transfection of HEK293T cells with cloned pro viral DNA as described.21 Concentrated HIV-1NL4-3 was prepared by 10 folds concentration through a 100,000 kD molecular weight cut centrifugal filter (Millipore). Levels of p24 in the viral supernatant were measured by ELISA using the PerkinElmer Alliance p24 antigen ELISA Kit (PerkinElmer). Viral titer (TCID50) was determined on Rev-CEM-Luc-GFP cells.18,19
CXCR4 Receptor Surface Staining
Rev-CEM-Luc-GFP cells were stained with PE-Cy5-labeled monoclonal antibody against human CXCR4 (Bio legend). Half a million cells were re suspended into 100µL of PBS + 0.1%BSA and then stained on ice for 30min with avoidance of light, washed with cold PBS +0.1%BSA twice and then analyzed on a FACS Caliber (BD Biosciences).
Drug Treatment and HIV Infection
Tadalafil and Sildenafil were purchased from Sigma. Tadalafil was dissolved in dimethyl sulfoxide (DMSO) (Sigma). Sildenafil was dissolved in H2O. For infection of Rev-CEM-Luc-GFP, 2 x 105 cells were pretreated with each of the above reagents for one hour at 37ºC. Cells were centrifuged at 300 x g for 5 minutes to remove the supernatant, and then infected with 200 µL of HIV-1 (250 ng p24) in the presence of the reagents. Cells were infected for two hours at 37ºC, washed, and then re-suspended into 1 mL medium with or without the drugs addition. Infected cells were cultured for three days and analyzed for GFP expression with flow cytometry on FACS Caliber (BD Biosciences). Propidium iodide (Fluka) was added (2 µg/mL) before flow cytometry to exclude dead cells. For HIV infection of PBMC, cells were pre-activated with PHA plus IL-2 for 24 hours. One million cells were treated with each of the reagents for one hour, and then infected with HIV-1 (250 ng p24) for 2 hours in the presence of the reagents. Infected cells were washed and re suspended in 1 mL of fresh medium with PHA plus IL-2, and the reagents were added back. Additional PHA plus IL-2 were added every 48 hours. Levels of p24 in the supernatant were measured on ELISA plates using the PerkinElmer Alliance p24 antigen ELISA Kit (PerkinElmer) or an in-house p24 ELISA kit. Briefly, each well of a plate was coated with capture antibody, incubated overnight at 37ºC, and then washed and blocked with blocking solution for 1 hour at 37ºC. Samples in plates were incubated for 2 hours at 37ºC, and then washed and incubated with biotin-labeled detection antibody for 1 hour at 37ºC. Plates were washed and incubated with avidin-peroxidase conjugate for 40 minutes at 37ºC followed by washing and incubation with tetra methyl benzidine (TMB) substrate buffer. Plates were kinetically read using an ELx808 automatic micro plate reader (Bio-Tek Instruments) at 630 nm.
We are grateful to the George Mason University Student Health Center for blood donation. This work was supported by the 2010 NYCDC AIDS Ride organized by M. Rosen, and in part by US Public Health Service grant 1R01MH102144 from NIMH to Y. W.
None.

©2015 Liang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.