Journal of
eISSN: 2373-6453


Research Article Volume 5 Issue 5
1Department, Environmental Research Divisions, National Research Center, Egypt
2Department of Medicinal and Aromatic Plants Research, National Research Center, Egypt
Correspondence: Mohamed Shaheen, Environmental Virology Laboratory, Water Pollution, Research Department, Environmental Research Divisions, National Research Center, Dokki, Giza, Egypt, Tel 201016710071
Received: February 13, 2017 | Published: July 14, 2017
Citation: Shaheen M, Mostafa S, El-Esnawy N (2017) Prevention of Coxsackieviruses and Rotaviruses Infections In Vivo with Methanol Extract of Dodonaea Viscosa . J Hum Virol Retrovirol 5(5): 00171. DOI: 10.15406/jhvrv.2017.05.00171
Human coxsackievirus B3 (CVB3) and rotaviruses (RV), RNA viruses, are major heart and intestine pathogens in children, respectively. In the current study, we determined the effect of methanol extract from the leaves of Dodonaea viscosa against CVB3 and RV replication in vivo. BALB/c mice were injected intraperitoneally with CVB3 at 105 TCID50 (50% tissue culture infective dose) or inoculated orally with RV at 106 TCID50, inducing acute myocarditis and gastroenteritis in infected mice. After 24 h of infection, the infected mice with CVB3 or RV were treated orally with the methanol extract of Dodonaea viscoasa leaves for 7 days. We found that the extract protected the infected mice from deaths and reduced the viral titers in heart tissues and fecal specimens of mice infected with CVB3 and RV, respectively. Interestingly, the extract showed markedly improved cardiac tissues and small intestinal lesion scores of infected mice with CVB3 and RV, respectively. Moreover, the extract reduced the elevation of the lactic dehydrogenase, Creatine kinase, and aspartate transaminase enzymes in serum of CVB3 infected mice. Also, the extract reduced the duration of diarrhea in the infected mice with RV, when compared with those in infected control. In our conclusion, the methanol extract could be used as strong therapeutic target for viral myocarditis and gastroenteritis caused by CVB3 and RV, respectively.
Keywords:Coxsackieviruses, Rotaviruses, In Vivo, Antiviral, Dodonaea Viscosa
AST, Aspartate Aminotransferase; BALB/c, An Albino, Laboratory-bred Strain of the House Mouse; BW, Body Weight; CAR, Coxsackie and Adenoviruses Receptor; CK, Creatine Kinase; DCM, Dilated Cardiomyopathy; DMEM, Dulbecco’s Modified Eagle Medium; EMEM, Eagle’s Minimum Essential Medium; GMK, Green Monkey Kidney; HW, Heart Weight; LDH, Lactate Dehydrogenase; NRC, National Research Centre; PFU, Plaque-Forming Unit; SD, Standard Deviation; V/C, Villi/Crypt
Human coxsackievirus B3 (CVB3), belonging to the genus Enterovirus within the family of Picornaviridae, is the most common pathogen of acute viral myocarditis in human, particularly children and adolescents.1-3 CVB3 can cause wide range of symptoms such as fever, gastrointestinal, headache, distress, muscle pain, and chest pain.4 Acute myocarditis due to CVB3 infection can result in chronic heart failure and dilated cardiomyopathy (DCM), which mostly requires cardiac transplantation.2,5 Also, CVB3 infections can cause serious diseases affecting the central nervous system or pancreas.6,7 Unfortunately, to date, there are no specific antiviral or therapeutic agents for treatment and/or prevention of the diseases resulted from CVB3 infections.8 Rotavirus (RV), belonging to the Reoviridae family, is the single most important cause of severe diarrheal disease especially in infant and young children, worldwide.9,10 RV is responsible for 114 million diarrhea episodes, 25 million clinic visits, 2.4 million requiring hospitalization, and over 500,000 deaths per year, usually children < 5 years of age, worldwide.11 To prevent RV infection, two rotavirus vaccines, RotaTeq and Rotarix, are currently licensed in many countries.12,13 However, the high cost of this vaccine production limits their application in developing countries.14 Therefore, cheap and effective drugs are urgent to control theses disease, particularly in developing countries. Several researchers, including our group, have been determined the antiviral activity of natural compounds from food and herbal extracts.15 Several natural compounds and herbal extracts have been reported to possess antiviral activity against coxsackievirus B3 and rotavirus infections in vitro and in vivo.16-24 So, they may be ideal sources for preventive and control these virus infections. Dodonaea viscosa, belonging to Spindaceae family, is known to contain several constituents such as flavonoids, sesquiterpenes, triterpenes, coumarins, monoterpenes, diterpenes, and steroids.25-28 The plant has been used to cure skin disease.29 Various biological activities such as antimalarial, antibacterial, anti-diarrheal, analgesic, antiulcer, antiviral, antidiabetic, anti-inflammatory, and antioxidant.19,30-36 activities are observed from this plant. In the present study we will evaluate the antiviral activity of methanol extract from Dodonaea viscosa leavesagainst coxsackieviruses and rotaviruses infections in vivo.
Plant collection
The leaves of D. viscosa were collected during May and June 2011, from the botanical garden of the National Research Centre (NRC), Giza, Egypt. The plant was kindly identified by Dr. Mona Marzok, researcher at NRC.
Extracts preparation
The methanol extract of D. viscosa leaves was prepared as described by Shaheen et al.22 In brief, after drying the leaves at room temperature, the leaves were dissolved in methanol and concentrated till dryness at 40oC in a rotary evaporator. The dried extract was weighted to estimate the percentage yield (concentration). To prepare the stock solution, 100 mg of the lyophilized extract was dissolved in 0.5 ml distilled Dimethyl sulphoxide (DMSO) then the volume was made up to ten milliliter with Eagle's minimum essential medium (EMEM) or Dulbecco's Modified Eagle Medium (DMEM). The stock solution was passed through membrane filtration (Millipore 0.45μm) for sterilization, and then stored in a refrigerator at 4oC until use.
Cell lines and viruses
Green monkey kidney cell line (GMK) with CVB3 were provided by Prof. Dr. Shubhada Bopegamage (National Reference Center of the Enter virus Laboratory, Faculty of Medicine, Slovak Medical University), under SAIA program. While Rhesus monkey kidney cell line (MA 104) and Simian rotavirus SA-11 were provided Prof. Mamta Chawla-Sarker (Department of Virology, National Institute for Cholera and Enteric Diseases, Kolkata, India), under INSA–JRD TATA program. The both cell lines were grown in EMEM and DMEM, respectively.
Virus titration
The GMK and MA 104 monolayers were infected with CVB3 and activated RV SA-11with trypsin (10 mg/ml) for 30 min at 37oC), respectively. The viral titers were estimated as TCID50/0.1 ml (50% tissue culture infectious doses/0.1 ml) by using standard Spearman Kärber formula.34 For our antiviral experiments, we used 104 log10 TCID50/0.1 ml and 106 log10 TCID50 for CVB3 and RV, respectively.
Cytotoxic effect of the extract in mice
Forty specific-pathogen-free BALB/c male mice, 1- 4 weeks old (10-15g), were obtained from the animal center of the NRC, were used in this experiment. The mice were divided into 5 groups (n =8 per group), four groups were treated with four different concentrations (400, 300, 200, 100 mg/kg/body weight) of the methanol extract via oral gavage for 7 days. Group (n=8) was treated with the same volume of phosphate buffer saline as negative control. The mice were monitored for deaths daily for 3 weeks.
Antiviral activity of methanol extract of D. viscosa on CVB3 and RV SA-11 infection in mice
For each virus, forty BALB male mice (1-4 weeks old) were used. The mice for each virus were distributed to five groups (8 mice per group). Among them, four groups were infected intraperitoneally with CVB3 (105TCID50/0.1 ml) or orally with RV (106 TCID50/0.1 ml) as fellow:
Infected group with virus/ inoculated orally with ribavirin at 10 mg/kg body weight as treated positive control.
For the group infected with CVB3: After 7 days of virus exposure, 4 mice from each group were subjected to blood collections from their eye socket and the activities of the lactic dehydrogenase, Creatine kinase, and aspartate transaminase enzymes were determined in the serum according to attached protocols found in purchase kits. From each mice, the heart tissues were collected to measure the ratios of body weight (BW) and heart weight (HW). Afterwards, the heart was divided into 2 parts; one to determine the viral titers of virus using plaque assay as described by Bishop and Koch.37 The second part of heart was subjected to histopathological examination to score the myocardial necrosis as follows: 0 = no infiltration or necrosis, 1= 1% - 25% infiltration or necrosis, 2= 26% - 50% infiltration or necrosis, 3= 51% - 74% infiltration or necrosis, and 4= 75% - 100% infiltration or necrosis.38
For the group infected with RV: During the period of treatment, we monitored the mortality, duration of diarrhea, and severity of diarrhea according to Shaw et al.39 based on 5 points as fellow: 0 = normal, solid and black, 1= soft brown, 2 = liquid brown, 3 = soft yellow, and 4 = liquid yellow. Also, the feces of each group were collected and pooled daily subjected to plaque assay to estimate the vial titers.40 At 7 days post-infection, four mice from each group were killed and the small intestine of each group were collected for histopathological examinations. The changes in small intestinal were scored as described by Kim et al.41 Based on the ratios of villi/crypt (V/C) which estimated as follows: 0 = normal, 1 = mild, 2 = moderate, 3 = marked and 4 = severe. The remaining mice in all groups were used for recording the deaths for additional one week.
Statistical analysis
Quantitative data were statistically represented as mean ± standard deviation (S.D.). Comparison between difference groups in the present study was done using One-way analysis of variance (ANOVA) test as comparison between more than two parametric groups with Dunnett and Duncan as multiple comparisons. A probability value (p value) less than or equal to (0.05) was considered significant. All statistical calculations and graphs were done using computer program SPSS (Statistical Package for Social Science) statistical program version (16.0) and Microsoft Excel program version (2010).
Cytotoxic effect of methanol extract of D. viscosa on mice
As depicted in (Table 1). The survival rate was 100% and no mortality was observed in the group of mice treated with the methanol extract at 100 mg/kg body weight. So, we used 100 and 50 mg/kg body weight as two safe doses in mice to be used in our antiviral experiments.
Group of Mice |
Concentrations/Kg Body Weight/Day |
Number Of Dead Animals |
Survival Rate |
Mortality Rate |
Control |
0 |
0 |
100% |
0% |
Methanolic extract of D. viscosa |
100 mg |
0 |
100% |
0% |
200 mg |
1 |
87.50% |
12.50% |
|
300 mg |
2 |
75% |
25% |
|
400 mg |
4 |
50% |
50% |
Table 1 Cytotoxicity results of methanolic extract of D. viscosa in vivo.
Antiviral Effect of Methanol extract of D. viscosa against CVB3 in Mice
Morbidity, mortality, and HW/BW ratios in infected mice: As shown in (Table 2). The mice treated with the extract at 100 and 50 mg/kg body weight showed less morbidity when compared with the infected control. Also, the extract at the both dosages protected the treated mice from deaths resulted from CVB3 infection, compared with the infected control. On the other hand, the HW/BW ratios were also significantly decreased in mice treated with the extract at 100 and 50 mg/kg body weight when compared with those in the infected control.
Group |
Morbidity (%) |
Mortality (%) |
HW/BW Ratios (Mean ± SD) |
Virus Titration(Log10 PFU/Ml, Means ± SD) |
Pathologic Scores (Mean ± SD) |
Normal control group |
0 |
0 |
4.21 ± 0.02 |
0 |
0 |
Infected group |
100 |
100% |
6.12 ± 0.03 |
6.42 ± 0.01 |
3.25 ± 0.10 |
Ribavirin (1 mg/mL) |
87.5 |
25% |
5.81 ± 0.02** |
3.45 ± 0.03** |
2.75 ± 0.35 ** |
Extract at 100 mg/kg |
12.5 |
0 |
4.23± 0.02** |
1.41±0.04** |
0.25 ± 0.01 ** |
Extract 50 mg/kg |
25 |
0 |
4.27 ± 0.04** |
1.86 ± 0.02** |
0.50± 0.03 ** |
P value a |
0.001 |
0.001 |
0.001 |
||
Table 2 Effect of Methanolic extract D. viscosa on morbidity, mortality, the heart index, virus titers, and pathologic scores after 7 days from inoculation of BALB/mice with CVB3.
Virus titers in heart tissues: Virus titers in the infected heart cells without treatment were 6.12 ± 0.03, cells, which reduced significantly to 4.23± 0.02 and 4.27 ± 0.04 in cells treated with the methanol extract at 100 and 50 mg/kg body weight, respectively (Table 2), indicating that this extract has potent antiviral effect against CVB3.
Effects of methanol extract on LDH, AST, and CK in infected murine serum: At 7 days post-infection, the activities of LDH, AST, and CK in the serum of the treated mice with the extract were very close to those in the normal control, which significantly increased in the infected mice without treatment (Figure 1).

Figure 1 Stained sections of the heart tissues from BALB/c mice (H&E, magnification 200 X): A: non-infected and non-treated mice; B: infected and non-treated mice; C: RBV mice; D: infected mice with RV and treated with the methanolic extract of D. viscosa at 100 mg/kg body weight; E: infected mice with RV and treated with the methanolic extract of D. viscosa at 50 mg/kg body weight.
Pathological evaluation
Scores of necrosis and filtration were 0.25 ± 0.01 and 0.25 ± 0.01 in the treated mice with the methanol extract at 100 and 50 mg/kg body weight, respectively. However, the necrosis and filtration were significantly increased to 3.25 ± 0.10 in infected mice without treatment (Table 2). On the other hand, the cell infiltration and necrosis were obvious in infected control, which greatly reduced in the mice treated with the extract at the both dosages at 7 days post-infection (Figure 2A-2E).

Figure 2 Histogram showing the effects of methanolic extract of D. viscosa leaves on the activities of AST, LDH, and CK in different groups of mice. Serum levels of LDH and CK were measured at day 7. n = 4 for each group. ** P < 0.01 versus the CVB3-infected group.
Antiviral Effect of Methanol extract of D. viscosa against RV in Mice
Mortality: The methanol extract at the both dosages protected the treated mice from the deaths resulted from RV diarrhea. However, 75 % of infected mice without treatment were dead, which decreased to 50 % in ribavirin treated group.
Severity and duration of diarrhea: The severity of diarrhea in the infected control was 4±0.03, which significantly reduced to 3.37±0.03 and 3.50±0.04 in the group of mice treated with the methanol extract at 100 and 50 mg/kg body weight, respectively. On the other hand, the extract recovered the treated mice from RV diarrhea in shorter time, compared to those that did not receive the methanol extract (Table 3).
Virus titers in feces of infected mice: As shown in (Table 3) the methanol extract greatly reduced the virus titers to 0.97 ±0.02 and 1.55±0.01 at 100 and 50 mg/kg body weight, respectively. Whereas, the virus titers in the feces of infected mice without receiving extract was higher (6.2±0.05).
Group of Mice |
Mortality |
Diarrhea |
Virus Titers (Log10pfu/Ml, Means± SD) |
Lesion Score (Means±SD) |
||
Number of mice developed diarrhea |
Severity (Diarrhea scores ±S.D.) |
Duration of diarrhea (Days± S.D.) |
||||
Normal control group |
0 |
0 |
0 |
0 |
0 |
0 |
Infected group |
75% |
8 |
4±0.03 |
6.2±0.05 |
6.50 ±0.05 |
3.75±0.01 |
Ribavirin 1 mg/mL) |
50% |
8 |
3.87±0.02** |
3.6±0.11** |
3.13±0.03** |
3.0±0.07** |
Extract at 100 mg/kg |
0 |
8 |
3.37±0.03** |
2.5±0.05** |
0.97 ±0.02** |
1.25±0.04** |
Extract at 50 mg/kg |
0 |
8 |
3.50±0.04** |
2.4±0.03** |
1.55±0.01** |
1.5±0.02** |
Table 3 Results of severity and duration of diarrhea, virus titers, lesion score of small intestine, and mortality in different groups of mice.
Histologic Examination: The lesion scores in treated mice were cores were greatly lower than those in infected control (Table 3). On the other hand, the lesions in the small intestine villi including dropsy and vacuolar degeneration were greatly reduced in mice treated with the extract at the both dosages, compared to those in infected control (Figure 3-3E).
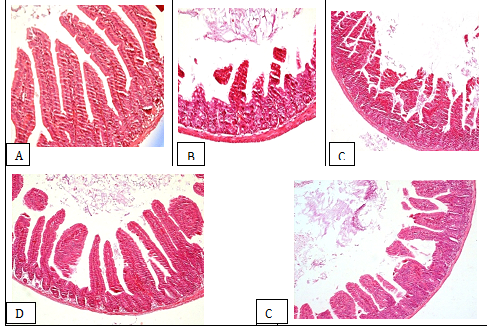
Figure 3 Stained sections of small intestine tissues from BALB/c mice (H&E, magnification 200 X): A: non-infected and non-treated mice; B: infected and non-treated mice; C: RBV mice; D: infected mice with RV and treated with the methanolic extract of D. viscosa at 100 mg/kg body weight; E: infected mice with RV and treated with the methanolic extract of D. viscosa at 50 mg/kg body weight.
The cytotoxic effect of ingestion of D. viscosa methanolic extract to mice was evaluated at various concentrations (100, 200, 300, and 400 mg/kg body weight), for seven days. Our results demonstrated that the extract is safe to mice at 100 mg/kg body weight and therefore we chosen 100 and 50 mg/kg body weight as two safe doses to be tested against CVB3 and RV infections. Our previous work suggested that the methanol extract of D. viscoasa has antiviral activity against CVB3 and RV in vivo.19 In this study, we demonstrated that this extract has also antiviral activity against both viruses in vivo, protecting the infected mice against viral myocarditis and gastroenteritis resulted from CVB3 and RV infections, respectively. To detect the protective effect of methanol extract against CVB3 myocarditis and RV gastroenteritis, Nancy strain of CVB3 and simian RV strain SA-11 were used to induce mild myocarditis and sever gastroenteritis in infected mice, respectively. In the treated mice daily with the extract after infection with CVB3, morbidity, virus titers, HW/BW, scores of pathologic, and mortality were significantly reduced than those in infected controls. In addition, the extract was significantly alleviated mononuclear cell infiltration and necrosis in the hearts of treated mice compared to infected controls. Moreover, the harmful effect of CVB3 myocarditis can lead to increase in the activity of AST, LDH, and CK enzymes.42 Interestingly, our extract kept the activities of these enzymes at normal values in the serum of treated mice, when compared with infected controls. These findings suggest that this extract has strong antiviral effect against CVB3 in vivo and can be used as therapeutic drug for viral myocarditis. Although, the mechanism by which the extract can inhibit CVB3 replication in vivo is not known, our previous work suggested that the methanol extract inhibited CVB3 replication in vitro by binding the extract to viral cased.19 Furthermore, it has been reported that coxsackie and adenoviruses receptor (CAR) serve as receptor for CVB3 infection.43 So, the methanol extract may be hindered the binding of virus to CAR, preventing its entry into host cells in the treated mice. On the other hand, oral inoculation of the methanol extract of D. viscosa after infection of newborn mice with RV showed lower mortality, rapid recovery from diarrheal disease, and lower virus titer than those in infected control. Moreover, the villi of the small intestine were higher in the treated group with the methanol extract when compared to mice that didn’t receive the methanol extract. Although we didn’t study how the extract can block RV infection in vitro but in our previous work we demonstrated that this extract suppressed RV replication in vitro by blocking the viral receptors.19 Saponin has be identified in the ethanol extract of D. viscosa leaves.44 Saponin has been reported to suppress RV infection by preventing virus-host attachment, reducing the mortality, severity, and duration of diarrhea in infected mice with RV.45
Moreover, we determined the presence of phenolic and flavonoid compounds in the methanolic extract of D. viscosa leaves, we find that the plant contains high percentage of total phenolics 15.58% /100mg and low percentage of flavonoid compounds (1.33% /100mg), data have not been shown. These compounds showed antiviral activity against several RNA viruses, among them CVB3 and RV.46,47 So, the inhibition of CVB3 and RV may be attributed to presence of such compounds in D. viscosa leaves. Finally, further studies are needed to isolate the bio-active compounds and to understand the exact mechanism by which these compounds can inhibit CVB3 and RV infections in vivo.
Our data showed that the methanol extract of D. viscosa leaves had potentially antiviral activity against coxsackievirus B3- induced myocarditis and rotavirus-induced gastroenteritis in mice, suggesting that this extract may represent a potentially novel therapeutic candidate for the treatment of viral myocarditis and gastroenteritis due to CVB3 and RV infections in human.
None.
None.

©2017 Shaheen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.