Journal of
eISSN: 2573-2897


Research Article Volume 5 Issue 3
Science Department, Knoxville Catholic High School, USA
Correspondence: Kelly P Kearse, Science Department, Knoxville Catholic High School, USA, Knoxville Catholic High School, 9245 Fox Lonas Road, Knoxville, TN, USA, Tel 865-560-0313, Fax 865-560-0314
Received: June 03, 2020 | Published: June 16, 2020
Citation: Kearse KP. The reddish color of bloodstains on the Shroud of Turin: investigation of two hypotheses. J His Arch & Anthropol Sci. 2020;5(3):101-107. DOI: 10.15406/jhaas.2020.05.00223
One of the most intriguing examples of aged bloodstains on an archeological textile are those found on the Shroud of Turin, a controversial linen cloth bearing the image of a man with wounds corresponding to scourging and crucifixion. Previous studies have demonstrated that the bloodstains test positive for various blood components including hemoglobin, albumin, and immunoglobulin, indicating they are not merely paint or pigment; however, as noted by many who have examined the cloth, the bloodstains are more reddish than would be expected for aged blood. It has been suggested that the reddish color may be a consequence of a residual coating of Saponaria, a softening agent used in the processing of ancient linen that contains hemolytic properties. Alternatively, the reddish color has been proposed to result from a high bilirubin content in the blood, transferred from a body that had undergone severe physical trauma. Here, both hypotheses are examined to assess the effects of such circumstances on bloodstain color over time. No effect of hemolysis on bloodstain color was observed, although, unexpectedly, it was found that a reddish color did persist in blood added to material that had been pre-treated with glycerin. Bloodstains with a high bilirubin content were not found to maintain a reddish color, regardless of the specific form of bilirubin present. The implications of these studies for bloodstain evaluation on the Shroud of Turin are discussed.
Keywords: Shroud of Turin, bloodstains, hemolysis, saponin, bilirubin
SAP, saponin
The Shroud of Turin is an approximately 14 x 3.5 feet linen cloth bearing the faint frontal and dorsal images of a man with reddish areas corresponding to wounds at the head, hands, feet and back. Although the Shroud has been heralded as the most studied archaeological object in the world,1 relatively little information exists about the character of the bloodstained areas that are present throughout the cloth, many of which soak through to the reverse side. The first endeavor to scientifically evaluate the molecular properties of the Shroud bloodstains began in 1973 by Frache and colleagues; hampered by ineffective solubilization methods, the presence of blood could not be ruled for or against.2,3 In 1978, the bloodstains were evaluated again as part of the STURP (Shroud of Turin Research Project) examination, involving over forty scientists of various disciplines with access to the Shroud for 120 continuous hours. Baima Bollone would also collect samples from bloodstained areas at this time. To date, this examination remains the most thorough investigation of the cloth, and is the main depository of the data related to the characterization of the bloodstains. It was concluded from such studies that the Shroud bloodstains were composed of real blood components and not the result of pigments or dyes.3‒10 Most recently, spectroscopic analysis has shown the bloodstains consist of methemoglobin, the deoxygenated form of hemoglobin expected for aged blood, corroborating previous findings on the hemoglobin type that is present.1,4 Whether human (or even primate) blood exists on the Shroud has not been fully resolved.11
An enigmatic feature of the Shroud bloodstains that has been commented on throughout the years is that they appear more red than would be expected for aged blood, some 700-2,000 years old (Figure 1). Several hypotheses have been put forth to explain the reddish color, including two major ideas that were proposed by scientists who were members of the STURP team. One hypothesis has never been independently tested, while the second has been examined by several investigators, but not without important and restricting caveats (see below). Previously, Rogers reported that addition of blood to saponaria-treated linen maintained a reddish color some thirty years later, compared to controls which were black; although, unfortunately, no photos were presented.12 Saponaria, or soapwort (also called “bouncing betty”), is a common plant whose extracts and leaves are used in the production of soaps and detergents. Rogers proposed that the reddish blood color on the Shroud was the result of a hemolytic residue being present on the cloth surface, which became known as the “hemolytic or Saponaria” hypothesis, although no independent tests have ever been performed to evaluate this idea.1,12 Such findings are potentially important in archaeological investigation as the color of bloodstains typically shifts from a bright red color to a brownish/black color over time. In surveying the scientific literature on the influence of hemolysis on bloodstain appearance in general, it was noted that relatively little information exists on this topic.
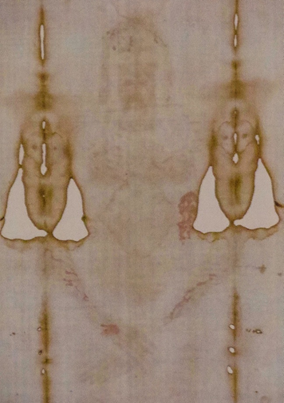
Figure 1 The frontal (ventral) image of the Shroud of Turin and accompanying bloodstained areas. Reddish bloodstains are visible in regions corresponding to the hair, forehead, side, arms, and wrist of the image. Photograph taken by the author in Torino, Italy during the 2015 exposition of the Turin Shroud. The photograph was taken at an approximate distance of 15 feet and no post-processing to enhance the color or contrast was used.
Another proposal for the reddish blood color on the Shroud is that it results from high amounts of bilirubin, an idea advocated by Adler, referred to as the “bilirubin hypothesis”.1,7,13 High bilirubin levels were suggested to be the result of excessive trauma, which led to extensive hemolysis (lysing of red blood cells), that, in turn, led to release of hemoglobin, followed by conversion into bilirubin. Alder specified that the form of bilirubin that was predominantly present was the unconjugated (unmodified) form, in which it exists immediately following creation from hemoglobin breakdown. Unconjugated bilirubin undergoes modification in the liver by addition (conjugation) of glucuronic acid, which makes it water soluble, a step which is necessary for effective excretion from the body. Adler proposed that the unconjugated levels in Shroud bloodstains would be elevated due to red blood cells being lysed at such a rapid rate. He suggested that rapid hemolysis would supersede the conjugation process, which would be unable to keep pace under such conditions, similar to what is observed with hemolytic anemia. In the examination of blood fibers taken from the Shroud, it was reported that “extraordinarily high amounts” of bilirubin were present, although no quantitation or estimated values were given and evaluation of specific forms was not feasible.3,13‒15 Several previous studies have evaluated the “bilirubin hypothesis”, although at very modestly increased levels(5-16x above normal) and with no distinction between the various bilirubin forms present, limitations that the authors themselves acknowledged.1,16,17 Indeed, in prior studies using blood from jaundiced patients, most of the bilirubin existed in the conjugated form, which is contrary to the specifics of Alder’s proposal.1,17 Additionally, previous studies have included the use modern anti-coagulants, a variable irrelevant to the ideas that the bloodstains originated from the Man of the Shroud (Jesus), or a medieval corpse. In the current report, experiments were performed to evaluate the basic premises of the “hemolysis (Saponaria)” and “bilirubin” hypotheses in relation to the effect on bloodstain color. The implication of these findings related to the Shroud bloodstains is discussed.
Blood
Human blood was obtained from healthy volunteers by the finger stick method using a Health Lancing device (CVS pharmacy, USA) fitted with a microlancet (CVS Pharmacy, USA. Blood was dropped onto Parafilm® M Laboratory Film (Bemis Company, Inc., Oshkosh, WI) and transferred to a 1.5 ml Eppendorf® microcentrifuge tube (Eppendorf, Hamburg, Germany) using a 10-100 microliter (ml) Eppendorf® micro pipettor (Hamburg, Germany), with the volume set at 100 ml; blood was then added to material as needed, using the same micro pipettor with the volume set at 20 ml. For freeze-thaw treatment, whole blood (200 ml) was placed in the freezer (-20oC) overnight, removed to thaw, and this cycle repeated 1-2 more times.
Linen and filter paper
Hanks of natural, unprocessed flax (Vavstuga, Shelburne Falls, MA) were woven into linen cloth by professional weaver Tess Farley (USA). Filter paper sources were Whatman filter paper 3mm (Whatman, Maidstone, Kent, UK).
Saponin preparation and treatment of textiles
Four sources of saponin were used in these studies: extracts from soapwort roots and leaves, prepared according to the methods of Budan et al.18 saponin on an applicator stick (In His Hands Birth Supply, Liberty Hill, TX); and saponin solution in 40% glycerol (HawaiiPharm, Honolulu, HI). For pre-treatment experiments, filter paper or linen was soaked overnight in saponin solution or solvent alone (control), removed and allowed to dry. In experiments using saponin on an applicator stick, saponin was mixed directly with the cell preparation prior to addition to linen or filter paper. Triton X-100 detergent (Consolidated Chemical & Solvents, Quakertown, PA) was used at a final concentration of 1%. Glycerol (glycerin) was purchased from CVS pharmacy (CVS, USA) and was diluted in distilled water at a final concentration of 10%-50%.
Hemoglobin release assay
Following treatment, phosphate buffered saline was added, samples were spun in a microcentrifuge, supernatants removed, and transferred to a new tube; supernatants were analyzed using a GENESYS 20 spectrophotometer at an absorbance of 543 nanometers, as described by Rodi et al.19
Bilirubin preparation
Unconjugated and conjugated bilirubin (Cayman Chemical Co., Ann Arbor, MI) were resuspended in chloroform or water, respectively, at a concentration of 10 mg/ml. The average range for total bilirubin in healthy adults is ~0.2-1.2 mg/dL. For these studies, an upper level of total was used as the reference for “normal” bilirubin levels at 1.0 mg/dL. Thus, in samples containing high bilirubin, the amounts were ~100x above the upper limit of total normal bilirubin and ~500x above the lower level of total bilirubin. In experiments using a mix of added unconjugated and conjugated forms, values refer to a combined 50/50 mixture of both forms.
Chemical induction of methemoglobin
NaNO2 (HiMedia Laboratories, Kennett Square, PA) treatment of whole blood or lysates was performed according to the method of Patton et al.20 NaNO2 was typically used at a concentration of 50 mM.
Animal models of hyperbilirubinemia
Whole blood from wild type and Gunn rats was obtained from RRRC, University of Missouri, USA. The Gunn rat contains a spontaneous mutation in the UDP-glucoronosyl transferase (UGT) gene and expresses high levels of bilirubin, approximately 7-10x that of normal, exclusively in the unconjugated form.21
Evaluation of the “hemolysis (Saponaria)” hypothesis: effect of hemolysis on bloodstain color
The hypothesis that hemolysis might influence bloodstain color on textiles was examined in two different ways: either by adding blood to material that had been treated with hemolytic agents (including saponin), or addition of red blood cell lysates to untreated material. Three different sources of saponin were used, including extracts prepared from roots or leaves from the soapwort plant, and saponin supplied on an applicator stick. Similar to studies by Rogers, blood was added to linen, and for this study also to Whatman filter paper, which provides a white background. As shown in Figure 2, blood was oxidized to a similar brown color on saponin-treated linen as untreated linen (Figure 2), visible within just twenty-four hours. Relatedly, when red cells were first lysed using either saponin or freeze-thaw methods, and then added to material, a reddish color did not persist over time (Figure 3). Digital analysis using RGB (red green blue) values was intentionally not presented in these studies as previous Shroud observations referred to a reddish color that was easily discernible by eye.
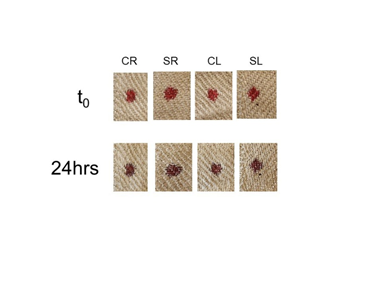
Figure 2 Bloodstain color on linen treated with saponin extracts. Linen samples were treated with saponin extracts purified from soapwort roots (SR) or leaves (SL) as described in Materials and Methods. Control extracts (CR, CL) were prepared in parallel and were treated identically as those containing saponin. Whole blood was added to the cloth and color evaluated immediately (t0) or 24 hours later.
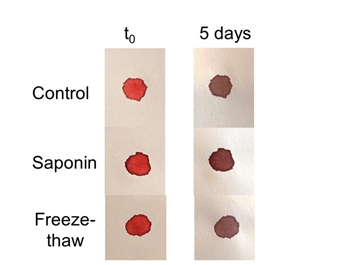
Figure 3 Effect of hemolysis on bloodstain color. Whole blood was either untreated (control), treated with saponin on an applicator stick (saponin), or subject to several cycles of freeze-thawing (freeze-thaw) to induce hemolysis. Samples were applied to filter paper and color evaluated immediately (t0) or 5 days later.
The effectiveness of saponin in hemolysis induction was verified by hemoglobin release, a standard assay used to measure red blood cell lysis (Figure 4); extracts from saponin roots were 94% as effective in hemolysis induction as Triton-X 100 detergent (used as the standard in this assay and set at 100%), (Figure 4A). Extracts from saponin leaves were somewhat less effective at hemoglobin release as root extracts, only 65% compared to Triton-X (Figure 4A). When cells were lysed using saponin on an applicator stick or by freeze-thaw methods, these were 75% and 95% as effectual as Triton-X, respectively (Figure 4B). Collectively, these data show that hemolysis was not associated with the persistence of red color in bloodstains over time. Interestingly, unlike previous results, when a fourth source of saponin was utilized (Saponin 4), a reddish color was seen to persist in bloodstains (Figures 5&6A), which was not observed in controls or linen pre-treated with Triton X-100 detergent (Figure 5). Saponin 4 was purchased in an aqueous form, containing ~ 40% glycerol. Most importantly, it was found that a similar effect occurred in groups that were treated with glycerol in the absence of saponin (Figure 6A). The reddish color associated with glycerol treatment persisted for at least 1 week, but was not observed after several months (Figure 6b). Taken together, these results indicate glycerol (glycerin)-treatment can influence the color of bloodstains on textiles, but that such effects are relatively short term.
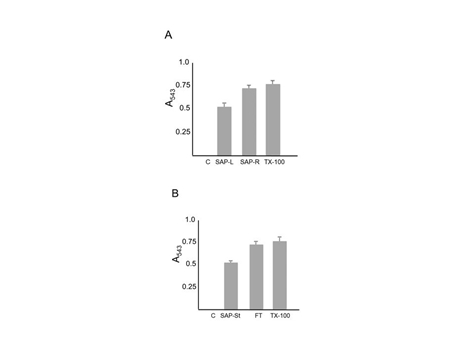
Figure 4 Hemolysis induction as measured by hemoglobin release. Samples were treated with the indicated substance and supernatants evaluated at an absorbance of 543 nm to measure release of hemoglobin, indicative of cell lysis. Panel A (Top): C = Control; SAP-L = Extract from saponin leaves; SAP-R = extract from saponin roots; Tx-100 = Triton X-100 detergent. Panel B (Bottom): C = Control; SAP-St = Saponin on an applicator stick; FT = Freeze Thaw; Tx = Tx-100 detergent. Each group was performed in triplicate; data are the mean plus standard deviation of absorbance at 543 nm.
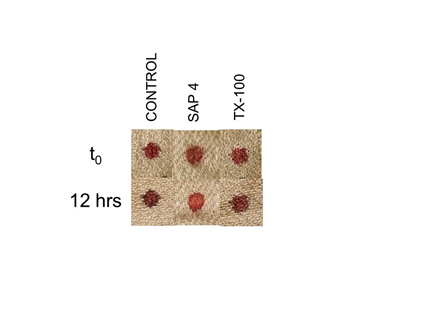
Figure 5 Effect of saponin solution 4 on bloodstain color. Whole blood was added to linen pre-treated with the indicated solutions and color evaluated. Control samples were treated with distilled water; SAP 4 is a commercially prepared aqueous solution of saponin (see Materials and Methods). TX-100 = Triton X-100 detergent.

Figure 6A Short term effect of glycerol on bloodstain color. Filter paper was pre-treated with distilled water (control), saponin 4 solution (SAP 4), or 40% glycerol solution (GLY). Whole blood was added and color evaluated at the time period indicated.
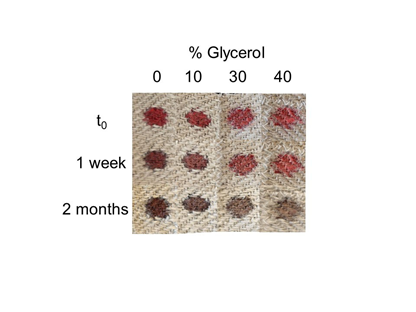
Figure 6B Long term effect of glycerol on bloodstain color. Linen was pre-treated with glycerol solution at the indicated concentrations; whole blood was added and color evaluated 1 week and 2 months later.
Evaluation of the “bilirubin” hypothesis: effect of bilirubin on bloodstain color
A central tenet of Adler’s bilirubin hypothesis is that mixing together methemoglobin plus high levels of bilirubin results in the color red. Methemoglobin is the deoxygenated form of hemoglobin that forms within minutes to hours upon the exposure of fresh blood to air. As blood dries, the iron present in the hemoglobin undergoes a conversion from the Fe2+ form to the Fe3+ form (creating methemoglobin), which cannot efficiently bind oxygen. Oxygen bound to the Fe2+ form of iron is what gives fresh blood its red color. Methemoglobin is formed during the natural aging of bloodstains, but may also be rapidly induced by chemical treatment with NaNO2.20 As shown in Figure 7, NaNO2 treatment effectively oxidized blood to a brownish color (Figure 7), which occurred using either whole blood or hemolysates (Figure 8). Importantly, as Figure 9 demonstrates, when high levels of unconjugated bilirubin are added to hemolysates containing methemoglobin the color remains brown, similar to what is observed in control groups (Figure 9). These results demonstrate that Adler’s prediction of methemoglobin plus high bilirubin in hemolysates yields a red color is not fulfilled.8,13 Relatedly, when blood containing high levels of unconjugated bilirubin was aged naturally, a brownish color was observed one month later, like what was seen in control groups (Figure 10). Similar findings were obtained when high levels of conjugated bilirubin were used (Figure 11), or a mixture of both bilirubin forms (Figure 12). Taken together, these data demonstrate that a reddish color does not persist in bloodstains containing high amounts of bilirubin, present in either unconjugated or conjugated forms. Such results are not in agreement with the main prediction of the bilirubin hypothesis: that a red color would be continuous under such conditions.
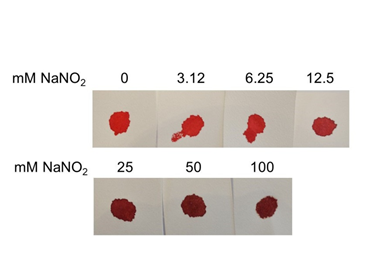
Figure 7 Dose response treatment of NaNO2 and bloodstain color. To chemically induce formation of methemoglobin, blood was treated with the indicated concentration of NaNO2 and transferred to filter paper. Color was evaluated immediately.
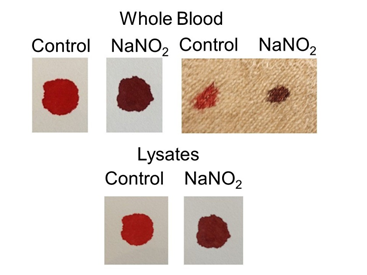
Figure 8 Effect of NaNO2 treatment on color of whole blood and lysates. Whole blood (top) or lysates (bottom) were treated with NaNO2 to induce methemoglobin and transferred to material. Color was evaluated immediately. In the groups shown on the top left, filter paper was used, on the top right, linen. In the bottom group (lysates), filter paper was used.
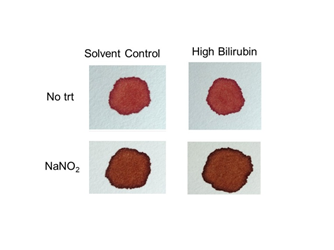
Figure 9Color of hemolysates containing methemoglobin and high levels of bilirubin. Lysates were treated with NaNO2 to induce formation of methemoglobin and mixed with unconjugated bilirubin corresponding to ~100-500x normal levels (see Materials and Methods). Samples were transferred to filter paper and color evaluated immediately.
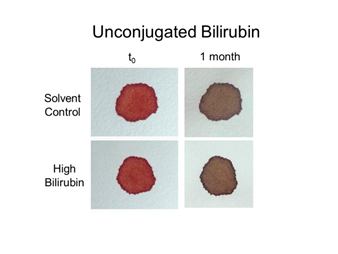
Figure 10 Effect of high bilirubin (unconjugated) on bloodstain color. Whole blood was mixed with unconjugated bilirubin corresponding to ~100-500x normal levels (see Materials and Methods), and samples transferred to filter paper. Color was evaluated immediately (t0) and 1 month later.
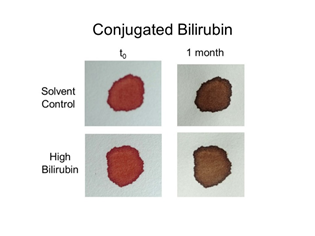
Figure 11 Effect of high bilirubin (conjugated) on bloodstain color. Whole blood was mixed with conjugated bilirubin corresponding to ~100-500x normal levels (see Materials and Methods), and samples transferred to filter paper. Color was evaluated immediately (t0) and 1 month later.

Figure 12 Effect of high bilirubin (unconjugated + conjugated) on bloodstain color. Whole blood was mixed with unconjugated and conjugated bilirubin corresponding to ~100-500x normal levels (see Materials and Methods), and samples transferred to filter paper. Color was evaluated immediately (t0) and 1 month later.
Finally, in extension of these results, experiments were performed using a rodent model that contains endogenously high levels of bilirubin in vivo, a condition referred to as hyperbilirubinemia. The Gunn rat contains a spontaneous mutation in the gene responsible for bilirubin conjugation; thus, all bilirubin in these animals exist exclusively in the unconjugated form, with 7-10x greater levels of bilirubin compared to normal, wild-type rats. As demonstrated in Figure 13, a reddish color did not persist in Gunn rat hemolysates over time (Figure 13). These findings support the above results with human blood containing increased amounts of exogenously added bilirubin.
The current report has investigated two major hypothesis regarding the reddish color of bloodstains on the Shroud of Turin, the “hemolytic (Saponaria)” hypothesis and the “bilirubin” hypothesis. As demonstrated in this report, exposure of red blood cells to hemolytic agents, including saponin, was not found to be associated with persistence of red color in bloodstains as originally suggested by Rogers.12 In the one case where saponin appeared to affect bloodstain color, this was shown to be a nonspecific effect due to the presence of glycerol (glycerin). Glycerol is widely used in the food, pharmaceutical, and cosmetic industries, and also in the freeze preparation of red blood cells,22,23 although this is the first instance that the author is aware of in which a color effect on bloodstains, albeit relatively short-term, has been noted. It is unclear in the original studies by Rogers what the basis was for the observation of persistence of reddish color in bloodstains over a time period of at least thirty years. Perhaps an unknown additive was present in the saponin extracts that were prepared; as relatively little information exists regarding the details of such studies, this remains indefinite. Rogers suggested that saponin-treatment was responsible for fluorescent profiles of the off-image of the Shroud, although the presence of saponin residue on the cloth has never been verified.12 If hemolysis was indeed responsible for the color effect as originally proposed, then induction of red cell lysis by any number of methods should result in a similar observation. The results in the current study provide evidence that this is not the case.
The bilirubin hypothesis is based on the suppositions that the Shroud bloodstains are the result of the cloth being wrapped around a body, specifically a body that had undergone severe trauma within the previous twenty-four hours, and the occurrence of extensive hemolysis and hemoglobin release, resulting in high bilirubin levels in the serum. A paucity of information exists regarding the correlation of bodily suffering with alterations in bilirubin levels or bloodstain color, although in fairness, this may be an issue that has not received much prior attention. Reportedly, Adler used a concentration of bilirubin some 500x above normal levels in the creation of a blood simulacrum to try to achieve a match with Shroud bloodstains for spectroscopic studies.24 It is unfortunate that no color experiments were documented during this time; such studies do not require copious amounts of blood (Adler’s simulacrum was created using three drops of blood from a fingerstick) and oxidation of blood to a brownish color occurs relatively quickly, within several days to a week after application. Although previous studies have examined the effect of increased bilirubin on bloodstain color in the context of the Shroud, the current report is the first to use much higher concentrations in the range suggested by Adler and to evaluate specific bilirubin forms specified by his hypothesis. Lesser (and higher) bilirubin concentrations were also evaluated with similar results, i.e. no effect of the persistence of red color in blood was observed (data not shown).
The suggestion that bilirubin content may contribute to the reddish color of the Shroud bloodstains is particularly interesting as bilirubin is notably light sensitive and unstable, a property which Adler himself emphasized regarding efforts to preserve the Shroud in the future.25 Several years ago, Prione made the interesting observation that bilirubin is also found in the red-orange fruits and flowers of certain plants, which is chemically indistinguishable from bilirubin expressed in humans.26,27 It is curious that plants, whose survival depends upon efficient collection of light would express bilirubin, a compound which is notoriously light sensitive. Conceivably, plants might express an associated molecule that helps confer bilirubin stability, although this is entirely speculation. Some might argue that an artisan may have used a plant with a red-orange pigment to mix with blood for coloration, and unknowingly also added bilirubin (in a relatively concentrated form) in the creation of the Shroud. As plant bilirubin and human bilirubin are chemically identical, these would be expected to react similarly in previous tests that were performed. On the other hand, if bilirubin levels were the result of physical trauma, this could be an important factor in bloodstain evaluation, in general, particularly in relation to victims of severe suffering or torture. Perhaps this is a consideration for forensic investigators and pathologists in the future, to note any anomalies (or not) in the color of aged blood under such circumstances, which could add to the reference database for examination of ancient textiles with bloodstains.
Why are the Shroud’s bloodstains reddish in color? The answer remains unsolved, although others have suggested they are exclusively composed of pigments,28 despite chemical and immunological data to the contrary,5,6,9,11 or are the result of real blood mixing with pigment that was used to stain the cloth.24 Lascio and colleagues have recently suggested that a combination of high bilirubin and uv exposure generates a color shift toward the red-yellow that is still measurable after four years.1 Similar to the various mechanisms that are proposed in the creation of the body image on the Shroud, further regimented investigation is needed to discern the molecular basis for the reddish bloodstains on this enigmatic archeological cloth.
In conclusion, the current study has evaluated two major hypotheses related to the reddish color of the bloodstains on the archaeological textile, the Shroud of Turin. By several measures, these data indicate that the reddish color does not result from hemolysis (the hemolytic or Saponaria hypothesis) or is due to increased levels of bilirubin in various forms (the bilirubin hypothesis).
As always, thank you to my wife Kathy, for everything.
None.
No conflicts of interest exist.

©2020 Kearse. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.