Journal of
eISSN: 2377-4312


Research Article Volume 4 Issue 2
1Instituto de Investigacion y Tecnologia en Reproduccion Animal (INITRA), Universidad de Buenos Aires, Argentina
2Catedra de Fisica Biologica, Universidad de Buenos Aires, Argentina
3Catedra de Histologia y Embriologia, Universidad de Buenos Aires, Argentina
4Catedra de Estadistica Analitica. Universidad de Buenos Aires, Argentina
Correspondence: Humberto Cisale, Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Av. Chorroarin 280, Ciudad de Buenos Aires, Argentina, Tel 5411 4524 8432
Received: August 25, 2016 | Published: November 22, 2016
Citation: Campi S, Jorge A, Lombardo D, et al. Yak (Bos Grunniens) sperm nuclei morphology, morphometry and DNA content. J Dairy Vet Anim Res.2016;4(2):272-275. DOI: 10.15406/jdvar.2016.04.00115
The aim of this study was to describe the morphology, morphometry and determine the DNA content of yak sperm nuclei (Bos Grunniens). Nuclear morphology observations were performed using light microscopy on slides stained with Feulgen reaction. Abnormalities percentages were determined: pyriform (1.2%), globose (0.2%), small (0.2%), elongated (0.1%). Morphometric measurements were made from digitized images of sperm nuclei stained with the Feulgen reaction. Mean values and standard deviations were obtained: area 21.98±2.60μm2, length 7.34±0.33μm, width 3.78±0.27μm, perimeter 88±19.26μm, roundness 1.27±0.04μm, elongation 1.95±0.11 and equivalent diameter 5.28±0.27μm. According to our knowledge this is the first time that haploid DNA content is determined. In this species 3.42pg value that was similar to that measured in other species of the family Bovidae and other higher mammals.
Keywords: yak, nuclei sperm morphology, morphometry, DNA, content
ML, milliliters; CP, centipoises; DNA, deoxyribonucleic acid; IOD, integrated optical density; HOS Test, hypoosmotic swelling test; SE, standard error; CV, coefficient of variation; µm, micrometers
The yak (Bos Grunniens) lives mainly on the Tibetan plateau in China; is also found in Mongolia, Russia, India, Nepal and Bhutan. The number of yaks has declined considerably in recent decades; this has gotten the attention of international conservation organizations for taking measures for the preservation of the species.1 Yak semen is milky white, with a pH from 6.4 to 6.7, a volume of 2 to 5ml and a concentration ranging between 7.5 to 16 x 108 sperm/ml. Its viscosity is 1.7cp, value lower than the one found in Bos Taurus (1.92cp).2 Its sperm morphology is similar to the one found in other bovines.2 The assessment of sperm morphology is an essential part of semen evaluation3 and has been traditionally included in the seminal routine analysis of different species, including: motility, viability, acrosome integrity and plasmatic membrane integrity and functionality.
In many species, a decrease in the percentage of normal sperm has been correlated with a decline in fertility,4‒6 and although it is useful for predicting, is highly useful supplementing with the determination of morphologic abnormalities of sperm nucleus7,8 that can be recognized using the Feulgen reaction.9,5 For the most objective determination of sperm parameters were developed employing calibration several computer software that determine mobility and sperm morphology.10 These analyses are used in humans, cattle, horses, sheep and alpacas, and have made possible to determine the size and shape of sperm (morphometry) in an objective and reproducible manner.11,12 Consulting the database Animal Size Data13 which brings together international publications of the last 50years on DNA content determination does not show results on the DNA content of yak (B. Grunniens). The aim of this study was to make a first approximation in the description of morphology and morphometry of normal sperm nuclei of frozen yak semen and to collect preliminary DNA content values in this species.
Frozen semen straws of yak from Semex -Canada®, which were thawed in a water bath at 37°C for 30 seconds were used. The content of the straws was placed in a tube preheated at 37°C. The following determinations were performed: live/dead, motility, morphological abnormalities, acrosomal abnormalities and membrane integrity and functionality. The percentage of live sperm was determined using eosin-nigrosine vital stain. Sperm motility was evaluated with ISASv1® software analysis. Membrane function was determined with the Hypoosmotic Swelling Test (HOS test).14 Sperm abnormalities were evaluated through a light microscopy with a 100x objective, and acrosome abnormalities using Giemsa stain.15 Sperm nuclei morphology was evaluated over three hundred sperm on slides stained with the Feulgen reaction, following the technique described by Ferrari et al.,16 for bovine sperm nuclei, with a total magnification of 1000x. The classification of nuclear morphologies was performed according to the parameters described by Barth & Oko.17
To determine nuclear morphometry, images of sperm nuclei stained with Feulgen reaction, using a high resolution digital camera Leica DC180 (Leica Microsystems Co., Wetzlar, Germany) with a 16 bits per channel sensitivity, were captured. One thousand frames were processed to obtain their respective binary images, measuring the following direct (area, length, width and perimeter) and indirect characters (roundness, elongation (length/width) and equivalent diameter) were obtained. Digital imaging software QWin Plus (Leica Microsystems Co.) was used. Measurements were made in individual and normal sperm nuclei, considering as such those that occurred in greater frequency in the population. All morphometric measurements were made by the same operator.
To determine the percentage of decondensed sperm nuclei Toluidine Blue stain was used.18 Three slides were done and 300 sperm nuclei were counted on each one. In normal sperm chromatin color ranged from green to light blue, whereas sperm with less compacted chromatin, color ranged from dark blue to magenta.19 Haploid sperm nuclei of normal cattle have steady DNA content (3.56pg). This reference value was used to determine the value in haploid sperm yak, according to Smith et al.20 Frozen bovine semen of four bulls were used, one of which was used to calibrate the equipment (calibration bull), and the remaining three for performing the calibration curve. To this purpose slides were divided into two areas, one corresponding to the yak semen and the other to the semen of each bull. All preparations were stained simultaneously with the Feulgen reaction to avoid experimental bias.21 The measurements were performed on sperm nuclei divided into three distinct areas: apical, middle and basal.
To quantify the observations and avoid subjectivity generated by human visual evaluation of the intensity of the brightfield mark by the Feulgen reaction, measures were performed digitally in the pictures obtained from stained slides by the Integrated Optical Density (IOD). Images were captured with a digital camera, model DC-180 (Leica Co.) mounted on an optical microscope and an image capture system supported by the program IM50 (Leica Co.). IOD values of the photographs were generated using the Leica Co QWin Plus software, by the grey intensity function - IOD. Based on the images obtained from the slides of the three bulls, a calibration curve was performed to dial different intensities. The maximum values of IOD were those corresponding to areas of lower DNA content and the lowest values were those belonging to the areas of greatest intensity of the reaction as revealed by a fuchsia color. Once the calibration curve was established, which consisted of eight arbitrary values designated by the digital measuring IOD set, the measurement of the samples of yak sperm nuclei were done in order to quantify and compare them.
To calculate the DNA content, the following formula was applied:
CVy, yak DNA content; CVb, Bull DNA content; DOIy, yak integrated optical density; DOIb, bull integrated optical density
![]()
Data were analyzed using descriptive statistics.
The results of the descriptive study of the sperm analyzed were: live sperm (84.5%), positive HOS test (75.0%), acrosome integrity (96.0%) and morphology normal sperm (83.3 %). The percentage of abnormal sperm tails (15.0%) was also determined. Of this percentage, 11.5% corresponded to whip tails and 3.5% remaining to coiled tails. Referring to the values of sperm motility, 34.5 ± 2.9% progressive motile sperm, 56.2 ± 6.0% nonprogressive motile sperm and 9.3 ± 0.8% static sperm were obtained. From nine hundreds of yak sperm nuclei analyzed, 98.3% had normal morphology. and the remaining 1.7% were abnormal with different morphologies (1.2% pyriform, 0.2% globose, 0.2% small and 0.1% elongated) (Figure 1).
Table 1 shows the results corresponding to morphometric determinations of yak sperm nuclei analyzed (area, length, width, perimeter, roundness, elongation, equivalent diameter and shape). The evaluation of the of yak sperm nuclei with Toluidine Blue staining showed that 100% of them had a normal condensation (light blue).
Table 2 resumes the results obtained with IOD in each zone that the sperm nucleus was divided.
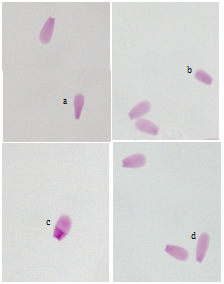
Figure 1 Examples of sperm nuclei illustrating various shapes of normal and abnormal morphologies of yak sperm nuclei. Magnification 1000 x.
Morphometric parameters |
Mean ± SE |
Area (μm2) |
21.98 ± 2.60 |
Length (μm) |
7.34 ± 0.33 |
Width (μm) |
3.78 ± 0.27 |
Perimeter (μm) |
19.26 ± 0.88 |
Roundness |
1.27 ± 0.04 |
Elongation (L/A) |
1.95 ± 0.11 |
Equivalent diameter (μm) |
5.28 ± 0.27 |
Shape (4πarea/perimeter2) |
0.77 ± 0.00 |
Table 1 Morphometric parameters of yak sperm nuclei stained with the Feulgen reaction using a Leica DC180 camera and Qwin Plus program
Mean ± SE: Mean ± Standard Error.
Elongation (length / width)
|
Calibration Bull |
Bull 1 |
Bull 2 |
Bull 3 |
Yak |
||||||||||
Apical |
Medial |
Basal |
Apical |
Medial |
Basal |
Apical |
Medial |
Basal |
Apical |
Medial |
Basal |
Apical |
Medial |
Basal |
|
Mean |
192.1 |
169.7 |
168.0 |
195.0 |
184.9 |
180.1 |
176.5 |
162.6 |
156.9 |
215.9 |
208.1 |
199.3 |
190.8 |
181.9 |
171.8 |
Standard Error |
16.2 |
18.5 |
14.9 |
11.3 |
10.9 |
9.7 |
16.5 |
19.3 |
15.6 |
10.1 |
10.3 |
10.2 |
12.6 |
14.0 |
10.1 |
Minimum |
130.5 |
129.8 |
120.3 |
169.0 |
152.5 |
146.7 |
136.0 |
125.0 |
126.5 |
189.0 |
183.8 |
174.5 |
161.8 |
152.0 |
145.3 |
Maximum |
245.0 |
230.0 |
236.0 |
229.3 |
215.7 |
206.3 |
221.0 |
211.5 |
202.0 |
237.0 |
235.0 |
222.0 |
236.3 |
239.4 |
195.0 |
Median |
191.7 |
174.8 |
168.3 |
193.6 |
185.0 |
181.6 |
175.0 |
160.5 |
154.5 |
214.2 |
207.5 |
198.0 |
190.0 |
182.0 |
172.0 |
CV |
8.4 |
10.9 |
8.9 |
5.8 |
5.9 |
5.4 |
9.3 |
11.9 |
10.0 |
4.7 |
4.9 |
5.1 |
6.6 |
7.7 |
5.9 |
Table 2 Values of Integrated Optical Density corresponding to the three areas in which was divided the sperm nucleus
CV: Coefficient of Variation; n=300
The values of progressive motility, morphological abnormalities and intact acrosomes obtained in the present study were similar to those found by other authors in yak frozen semen.1 The evaluation of sperm morphology is a major component of the semen evaluation. Abnormal nuclear morphologies that were found in this study are similar to those found in another species.17,22 The prevalence of these is similar to those found in bovine semen by other authors.22
Morphometric measurements were made on yak normal sperm nuclei from frozen sperm stained with the Feulgen reaction. Shape sperm nucleus through the relationship 4π area/perimeter2 and length/width was also estimated. Although most of the sperm head is occupied by the nucleus, all values found were lower than those found in sperm heads of fresh semen of cattle, horses, goats, Iberian red deer and rabbits.23,24 This decrease may be a consequence that the cryopreservation process would produce a reduction of the morphometric measurements.25 The DNA value (3.42pg) obtained was similar to that measured in other species of the family Bovidae and other higher mammals,13 Since variations in the DNA content are rare among individuals of the same species, and even within the same individual with normal and abnormal nuclear morphologies have no significant differences,26 the result could be considered within the average for the species. Furthermore, karyotypes of bull and yak are similar in morphology and number of chromosomes. Both have 60 chromosomes, 58 acrocentric autosomes and submetacentric sex chromosomes X and Y.27
In this paper the value of haploid DNA content from Bos Grunniens were obtained for the first time, according to our knowledge. Also, mean values of morphometric parameters of the sperm nucleus were determined using methodologies employed in other species, were also found to be suitable for the analysis of sperm nucleus of frozen semen of Bos Grunniens. These results could enrich biodiversity and evolutionary studies and contribute to the knowledge of semen quality in this species.
This research was conducted in Argentina and supported by grants from the Universidad de Buenos Aires UBACyT 2002010010081. We thank Ing. Agr. Mariano Etcheverry and Biogenetics Argentina for kindly providing the yak samples used in this study.
Author declares that there is no conflict of interest.

©2016 Campi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.