Journal of
eISSN: 2377-4312


Case Report Volume 11 Issue 1
1Faculdades Qualittas, São Paulo, São Paulo
2Department of Veterinary Medicine, University of Northern Parana, Londrina, Brazil
3Faculdade de Medicina Veterinária, Universidade de Sorocaba, Sorocaba, São Paulo
Correspondence: Mauro José Lahm Cardoso, Professor at College Qualities, Padre Leonel France Street, 641, Jardim Leonor, Campinas, São Paulo, Brazil
Received: September 16, 2022 | Published: September 23, 2022
Citation: Cardoso MJL. Use of omega-3 as adjunctive therapy in the control of hyperlipidemia in metabolic and endocrine diseases in dogs. J Dairy Vet Anim Res. 2022;11(1):20-25 DOI: 10.15406/jdvar.2022.11.00310
Background: Omega-3 from fish is commonly recommended for the control of hyperlipidemia, however, there are few studies in dogs proving its efficacy, and no studies have compared doses of omega-3 in patients with severe hyperlipidemia.
Hypothesis: The dose of 75mg/kg of omega 3 is sufficient to reduce serum levels of triglycerides and cholesterol in different metabolic and hormonal diseases.
Keywords: dyslipidemia, cholesterol, triglycerides, obesity, diabetes mellitus, Cushing’s syndrome, hypothyroidism
VLDL, very low density lipoprotein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALA, -linoleic acid; PLS, lipoprotein lipase;
Dyslipidemia or hyperlipidemia is a circulatory alteration or increase in lipids in general (triglycerides-TG, cholesterol, and fractions) and/or lipoproteins, which have the function of transporting lipids of endogenous and exogenous origin in the blood and lymphatic circulation.1–3
The diagnosis of hyperlipidemia is confirmed by the elevation of serum or plasma cholesterol (hypercholesterolemia) and/or triglycerides (hypertriglyceridemia) after fasting for 10-12hours. Hyperlipidemia can cause steatosis, cholestasis, and/or vacuolar liver disease and, consequently, an increase in liver enzyme activity (ALT, FA, GGT). Hypercholesterolemia and hypertriglyceridemia can be classified as mild, moderate, and severe.1,6,10,21,23
Hypertriglyceridemia can be caused by increased production of chylomicrons (CM, dietary excess of lipids and carbohydrates), ineffective removal of CM from the circulation, increased production of very low density lipoprotein (VLDL), and excessive release of VLDL. Hypercholesterolemia is caused by increased production of low-density lipoprotein (LDL) precursor, and reduced release of LDL and high-density lipoprotein (HDL).1,6,10,20
Hyperlipidemia can be classified as primary or secondary. In dogs, the primary causes are idiopathic hyperlipidemia in the Miniature Schnauzer, Briards hypercholesterolemia, and idiopathic hypercholesterolemia. Secondary hyperlipidemia can occur due to high-fat diets, endocrinopathies, including Diabetes Mellitus, hypothyroidism, hyperadrenocorticism, the use of glucocorticoids, obesity acute pancreatitis, cholestasis, liver failure, and protein-losing nephropathies.4,9,15–23,26
Complications of hyperlipidemia include hepatobiliary disorders (steatosis, cholestasis/cholecystitis, vacuolar liver disease, and mucocele) seizures, insulin resistance, pancreatitis, uveitis, acute blindness, and retinal and corneal lipemia, and those of hypercholesterolemia include atherosclerosis.6,7,9,10,23,27
Theapy for the control of hyperlipidemia is recommended when triglyceride and cholesterol values are classified as severe. Therapy includes treatment of the underlying disease, a fat-restricted diet with increased levels of fiber and protein, and carnitine supplementation. The most commonly used drug therapy is made up of fibrates and omega-3 fatty acids and fish oil.1,3,10–14,19,22,28
There are several omega-3 long-chain polyunsaturated fatty acids (PUFAs), the most common being a-linoleic acid (ALA), eicosatetraenoic acid (EPA), and docosahexaenoic acid (DHA). PUFAs decrease intestinal chylomicron (CM) absorption, liver TG synthesis, TG incorporation into VLDL particles, TG-rich VLDL secretion, and hepatic lipogenesis, and increase fatty acid β-oxidation, promoting greater degradation of the VLDL-rich molecule and greater secretion of cholesterol by bile. In addition, they increase the activity of serum lipoprotein lipase (LPS), which has the function of promoting the hydrolysis of TG contained in VLDL and CMs, leading to greater plasma removal. In diabetes mellitus, hypothyroidism, and hyperadrenocorticism, the activity of LPS is reduced.2,12
In the literature, the doses of omega-3 described for dogs are 1g/4.5kg, and 220mg/kg/day, the equivalent of 66mg/kg of DHA and EPA. The objective of the current study is to use a lower dose than this in patients with mild to moderate hyperlipidemia of endocrine or metabolic origin, in addition to identifying possible side effects from the therapy.1,13
The study was carried out from 2018 to 2020 in 67dogs with hyperlipidemia of different degrees of primary and secondary severity (Diabetes Mellitus, Hyperadrenocorticism, Obesity and Hypothyroidism) treated at private Veterinary Clinics in different states of Brazil. The research project was approved by the Ethics Committee and all owners signed the Free and Informed Consent Form.
Patients with pancreatitis, mucocele, cholecystitis, and diabetic ketoacidosis were excluded from the study, as well as patients receiving phenobarbital, and glucocorticoids for the treatment of chronic diseases such as autoimmune, hypoadrenocorticism, or neoplasms. No patients who were receiving bezafibrate or who had taken it in the previous 30days participated in the study.
The study was double blind; the choice of omega-3 dose was random and blinded. Patients received omega-3once a day for 60days associated with the diet and treatment for the underlying disease prescribed by the Veterinarians responsible for conducting the clinical case. Fish omega-3 capsules containing DHA and EPA available in the national market were used (Ograx®-Avert; Omega 3SE®-Ventil).
Patients were divided into three groups: Group 1 (G1): Omega-3 at a dose of 75mg/kg; Group 2 (G2): Omega-3at a dose of 100 mg/kg, Group 3 (G3): Omega-3 at a dose of 200mg/kg. The groups were subdivided according to severity into mild/moderate and severe hyperlipidemia and also according to the underlying disease. Serum cholesterol concentrations between 300-750 were considered as mild to moderate and above 750md/dl as severe and serum triglyceride concentrations between 150-1000mg/dl were considered as mild to moderate and above 1000mg/dl as severe.1,6,10,21
Exams were collected at three different moments, distributed as follows: moment zero (MO) before starting treatment, moment 1 (M1) after 30days of treatment, moment 2 (M2) after 60days of treatment. At MO, serum measurements of triglycerides, cholesterol, FA, GGT, ALT, urea, creatinine, protein, albumin, and glucose were performed, in addition to abdominal ultrasound, rapid canine specific lipase test (Snap-canine), and urinalysis. Specific tests were also performed for the diagnosis of endocrine diseases, such as the ACTH stimulation test and/or a suppression test with a low dose of dexamethasone to measure cortisol, fructosamine, endogenous insulin, and thyroid profile (TT4, FT4, and TSH). At M1, only triglycerides and cholesterol were measured. At M2, serum measurements of triglycerides, cholesterol, FA, GGT, ALT, urea, and creatinine were performed. Exams to monitor the underlying diseases were carried out whenever necessary. Patients were asked to fast for 10 to 12hours for the triglycerides measure.
To evaluate the effect of continuous administration of omega-3 on biochemical and hematological parameters, the initial values were compared after 30and 60days by the Wilcoxon test, considering the dose and the brand as independent variables, and the levels of cholesterol, triglycerides, urea, creatinine, FA, GGT, and ALT as dependent variables. The effect of brands was evaluated by the Mann Whitney paired test, considering the brand as an independent variable and the differences in biochemical and hematological parameters at 30days (for cholesterol and triglycerides) and at 60days of treatment (for all other variables) as dependent variables.
The effect of the doses was evaluated by the KruskalWalli’s test, considering the dose as an independent variable and the differences in the biochemical and hematological parameters at 30days (for cholesterol and triglycerides) and at 60days of treatment (for all other variables) as dependent variables.
The animals were also categorized according to the degree of disease severity into two groups: (1) mild/moderate, and (2) severe, and according to the pathology, also into two groups. (1) Diabetes and (2) Cushing's syndrome. In each group, the effect of doses was evaluated by the Kruskal Wallis test, considering the dose as an independent variable and differences in triglyceride and cholesterol levels at 30and 60days of treatment as dependent variables. All analyses were performed using the Statistical 13.2 program with significance ≤ 5%.
In total, 67dogs were included, from six private veterinary clinics in the state of Paraná (1), São Paulo (2), Rio de Janeiro (2), and Santa Catarina (1), all located in Brazil. Of these 67dogs, interruption and/or withdrawal of therapy occurred in 35.8% (24dogs). The 43dogs included patients with Hyperadrenocorticism (13), Diabetes Mellitus (8), Obesity (8), Primary Dyslipidemia (8), Hypothyroidism (5), and Diabetes Mellitus associated with Hyperadrenocorticism (1). Brand 1 was administered to 14 dogs and brand 2 to 29dogs. Of the 43dogs, 27 were females (25 neutered and 2 not neutered) and 16 were males (12 neutered and 4 non-neutered), being Mixed Breed (MB) (11), Lhasa Apso (6), Miniature Schnauzer (5), Shi-Tzu (5), Yorkshire terrier (4), Poodle (3), Maltese (2), Pinscher (2), German Spitz (2), Dachshund (2), and Scottish Terrier (1), with weight ranging from 2 to 39.8kg (mean 11.6kg).
Nine (13.4%) of the 67dogs that started therapy (3/9 with hyperadrenocorticism, 2/9 with Diabetes Mellitus, 1/9 with Diabetes Mellitus and Hyperadrenocorticism, 1/9 with obesity, 1/9 with hypothyroidism, and finally 1/9 with primary hyperlipidemia, one Schnauzer) presented the development of gastrointestinal signs such as vomiting and diarrhea, with seven receiving a dose of 200mg/kg and two receiving a dose of 100mg/kg. One of the patients, with controlled diabetes mellitus had pancreatitis, confirmed by abdominal ultrasound and canine-specific lipase SNAP test (Index®). The owners of five (7.5%) dogs withdrew from treatment with omega-3 due to the odor of the product, of which two dogs were obese, two had Hyperadrenocorticism, and one had primary hyperlipidemia; three of these were receiving the dose of 200mg/ kg. The odor of fish oil, particularly in animals given high doses of omega-3 fatty acids, was considered unacceptable by some owners, which was also observed in the present study. It should be noted that the dogs that received the lowest dose (75mg/kg) demonstrated no side effects. The total occurrence of side effects was 20.9%, including the odor that led to withdrawal from the treatment. The gastrointestinal signs observed were not necessarily due to the use of omega-3.12,13,28
Six clients did not return with their dogs at 30 (2) or 60 (4) days, of these, three dogs were obese, one was hyperadrenocorticism, one had diabetes mellitus, and one had hypothyroidism. Two patients with hyperadrenocorticism and diabetes mellitus did not return, as they died from thromboembolism and mucocele, respectively. The other dogs did not provide the reason for the withdrawal or discontinuation of therapy. In four dogs, omega-3 was switched to bezafibrate, as the hypertriglyceridemia remained severe and pancreatitis developed in two patients and mucocele and cholecystitis occurred in one patient each. These complications may be associated with a high increase in triglycerides, although there is a description in the literature that both omega-3 fatty acids and bezafibrate promote improvement in inflammatory processes.7,11
All patients received treatment for the underlying disease, including triclosan for hyperadrenocorticism, levothyroxine sodium for hypothyroidism, NPH insulin/detemir/can insulin in the presence of Diabetes Mellitus, and a calorie-restricted/increased fiber and protein diet for obese dogs and those with primary hyperlipidemia. The diets recommended for the patients who completed the study were Hill’s® w/d (5/43) and r/d (3/43); Premier® Obesity (4/43) and Diabetes (3/43); Royal Canin® Low Fat (3/43), Diabetic (2/43), and Satiety (5/43), Pro Plan® Veterinary Diets DM (2/43), Vet Life® Obesity and Diabetes (5/43), Equilibria® Obesity and Diabetes (5/43), and natural/homemade food (6/43).
The 43 dogs that completed the study also received other medications for the control of adjunctive diseases or complications, including Uros deoxycholic acid (31), S-Adenosyl-Methionine-SAME (17), antibiotics (15-enrofloxacin, amoxicillin/clavulanic, marbofloxacin, metronidazole, cephalexin), antiemetics (12-maropitant and ondansetron), amlodipine (11), omeprazole (10), benazepril (9), products containing collagen/chondroitin/glycosaminoglycans (9), pimobendane (5), clopidogrel (5), non-steroidal anti-inflammatory (5), silymarin (5), vitamin complex containing iron and vitamin B (3), gabapentin (3), enoxaparin (2), tramadol (2), furosemide (2), spironolactone (1), Apoquel® (1), Cytopoint® (1), cyclosporine (1), and pancreatic enzymes (1). These medications did not interfere with the responses to the different doses of omega-3 used, as the responses to the decreasing doses did not show any difference between the three groups studied Figure 1 & Table 1-3.1–3
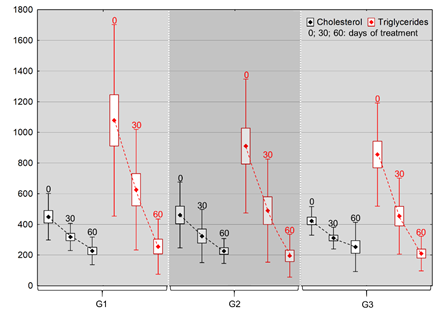
Figure 1 Mean values (points), with deviation (bars) and standard error (lines) of cholesterol and triglyceride levels after 30 and 60days of continuous omega-3 administration, categorized by dose (G1: 75mg/kg; G2: 100mg/kg, G3: 200mg/kg).
Brand 1 |
Brand 2 |
|||||
|
G1 |
G2 |
G3 |
G1 |
G2 |
G3 |
Cholesterol M0 |
412.80 b±200.59 |
379.60 b±193.81 |
420.40 b±97.38 |
469.11 c±126.71 |
505.89 c±222.97 |
423.40 c±96.44 |
Cholesterol M1 |
334.20 b±127.39 |
286.20 b±148.00 |
281.20 a±94.40 |
308.11 b±65.28 |
343.44 b±191.07 |
325.00 b±55.21 |
Cholesterol M2 |
191.20 a±43.74 |
174.80 a±48.87 |
257.00 a±62.41 |
246.22 ab±104.36 |
253.89 a±83.83 |
250.60 ab±195.85 |
TG M0 |
1385.00 c±946.01 |
979.6 c±342.97 |
1039.20 c±374.75 |
907.89 c±308.41 |
871.89 c±496.93 |
763.30 c±292.38 |
TG M1 |
745.40 b±579.83 |
544.00 b±396.02 |
530.40 b±391.87 |
559.22 b±263.58 |
458.89 b±319.50 |
415.20 b±148.79 |
TG M2 |
319.80 a±252.74 |
270.20 a±208.59 |
207.60 a±120.67 |
218.56 a±128.47 |
152.56 a±65.41 |
209.90 a±115.76 |
Urea M0 |
40.00 a±44.75 |
57.80 a±76.28 |
67.40 a±48.49 |
56.22 a±45.00 |
37.49 a±34.65 |
52.00 a±33.99 |
Urea M1 |
34.00 a±10.68 |
34.20 a±5.76 |
52.00 a±31.98 |
29.11 a±8.36 |
32.00 a±6.91 |
29.40 a±7.83 |
Creatinine M0 |
1.37 a±1.47 |
1.03 a±0.28 |
1.61 a±0.85 |
1.36 a±0.71 |
1.02 a±0.71 |
1.36 a±0.81 |
Creatinine M2 |
0.90 a±0.34 |
0.95 a±0.27 |
0.88 a±0.32 |
0.89 a±0.32 |
0.86 a±0.31 |
0.98 a±0.45 |
FA M0 |
589.00 a±339.18 |
614.60 b±220.21 |
734.60 b±273.99 |
604.67 b±405.87 |
911.00 b±655.24 |
1329.40 b±1064.45 |
FA M2 |
270.20 a±81.56 |
182.60 a±95.20 |
185.40 a±54.67 |
183.00 a±90.66 |
218.44 a±111.62 |
188.40 a±110.38 |
GGT M0 |
22.88 b±19.31 |
6.74 a±3.70 |
4.14 a±0.97 |
7.37 b±3.63 |
7.70 b±4.37 |
14.30 b±22.79 |
GGT M2 |
7.78 a±7.53 |
4.30 a±3.17 |
7.02 a±6.09 |
3.47 a±1.72 |
4.00 a±2.65 |
3.68 a±2.39 |
ALT M0 |
178.80 b±135.00 |
126.60 a±68.60 |
301.80 b±176.77 |
232.33 b±88.09 |
264.33 b±141.92 |
270.10 b±180.44 |
ALT M2 |
52.40 a±25.32 |
115.20 a±90.24 |
107.80 a±85.33 |
91.34 a±78.66 |
76.78 a±30.03 |
55.60 a±27.52 |
Table 1 Means and standard deviation of cholesterol, triglycerides, urea, creatinine, FA, GGT, and ALT before treatment initiation (MO), and 30 days (M1) and 60 days (M2) after continuous omega-3 administration categorized by brand and dose
|
Brand |
G1 |
G2 |
G3 |
Cholesterol – M1 |
1 |
78.60 Aa± 111.18 |
93.4 Aa± 117.33 |
139.2 Aa± 91.34 |
2 |
161.00 Aa± 126.21 |
162.44 Aa± 173.65 |
98.40 Aa± 60.14 |
|
Cholesterol – M2 |
1 |
221.60 Aa± 215.97 |
204.80 Aa± 171.15 |
163.40 Aa± 148.61 |
2 |
222.89 Aa± 189.30 |
252.00 Aa± 190.31 |
172.80 Aa± 189.59 |
|
TG – M1 |
1 |
639.60 Aa± 434.65 |
435.60 Aa± 297.26 |
508.80 Aa± 220.88 |
2 |
348.67 Aa± 84.38 |
413.00 Aa± 287.67 |
348.10 Aa± 172.29 |
|
TG – M2 |
1 |
1065.20 Aa± 726.06 |
709.40 Aa± 228.20 |
831.60 Aa± 273.22 |
2 |
689.33 Aa± 221.53 |
719.33 Aa± 445.09 |
553.40 Aa± 211.36 |
|
Urea – M2 |
1 |
6.00 Aa± 52.80 |
23.60 Aa± 79.31 |
15.40 Aa± 38.51 |
2 |
27.11 Aa± 46.84 |
5.49 Aa± 33.17 |
22.60 Aa± 36.35 |
|
Creatinine – M2 |
1 |
0.47 Aa± 1.36 |
0.08 Aa± 0.44 |
0.73 Aa± 0.91 |
2 |
0.47 Aa± 0.80 |
0.15 Aa± 0.88 |
0.38 Aa± 0.77 |
|
FA – M2 |
1 |
318.80 Aa± 281.12 |
432.00 Aa± 139.37 |
549.20 Aa± 222.45 |
2 |
421.67 Aa± 374.99 |
692.56 Aa± 593.30 |
1141.00 Aa± 994.70 |
Table 2 Means and standard deviation of decreases (initial value minus value at 30 or 60 days) in cholesterol, triglycerides, urea, creatinine, and FA levels after 30 and 60 days of continuous omega-3 administration categorized by brand and dose
|
Dose (mg/kg) |
Severity |
|
|
Mild/Moderate |
Severe |
|||
TG – M1 |
G1 |
674.50 a± 331.93 |
286.13 a± 48.82 |
|
G2 |
681.80 a± 288.63 |
276.22 a± 139.27 |
||
G3 |
604.00 a± 211.88 |
300.50 a± 82.85 |
||
TG – M2 |
G1 |
1179.00 a± 560.68 |
557.00 a± 90.35 |
|
G2 |
1106.60 a± 336.36 |
498.67 a± 138.28 |
||
G3 |
943.60 a± 199.63 |
497.40 a± 122.74 |
||
Table 3 Means and standard deviation of decrease (initial value minus value at 30 or 60 days) in triglyceride levels after 30 and 60 days of continuous omega-3 administration, categorized according to dose and disease severity
Normalization of the mean cholesterol value occurred in the 43dogs that completed the study and the normalization of cholesterol values was verified in 76.6% of the 43dogs. Only eight dogs had values above 270mg/dl at the end of the study (3 from G1, and 2 each from G2 and G3) and only one dog with Diabetes Mellitus continued to present a severe increase in cholesterol (790mg/dl) after 60days of treatment, despite receiving the highest dose of omega-3. The average value of triglycerides after 60days was 219 mg/dl. There was a reduction in triglycerides in 20dogs (46.5%) and, of these, 19had mild hypertriglyceridemia (<400mg/dl) and 4 dogs had moderate hypertriglyceridemia (400 -1000mg/dl). Of the dogs that showed a moderate increase, two were in G1, one in G2, and one in G3. The patient with the highest serum triglyceride concentration (728mg/dl) at the end of the study had primary hyperlipidemia. This result may suggest that in severe primary hyperlipidemia, the use of bezafibrate may promote normalization of triglyceride values more quickly than the use of omega-3 from fish, as described in a previous study, more recently, with the use of fenofibrate.3,14
The laboratory parameters regarding the brand of omega used are described in Table 1. Regardless of the dose, the cholesterol of animals treated with brand 2 decreased after 30days of administration, showing a statistically significant difference between the three moments in G1 (p>0.05). In the group of animals treated with brand 1, the decrease in cholesterol occurred after 30days for the 200mg/kg dose, and after 60days for the 75mg/kg and 100mg/kg doses.1 with differences between times and doses (p>0.05). Regardless of the dose and brand, the triglyceride level decreased after 30days and continued to decrease after 60days of treatment.1 Regardless of dose, it was observed that the longer the treatment time, the greater the reduction in triglyceride and cholesterol concentrations, a similar result had been described in healthy dogs receiving high doses of PUFAs rich in omega-3 Table 4.
|
Disease |
G1 |
G2 |
G3 |
|
Cholesterol – M1 |
DM |
167.25 a± 14.89 |
304.00 a± 248.70 |
114.00 a± 62.23 |
|
CS |
145.20 a± 210.62 |
46.67 a± 103.68 |
116.80 a± 55.17 |
||
Cholesterol – M2 |
DM |
331.50 a± 158.89 |
402.33 a± 181.33 |
234.50a± 221.32 |
|
CS |
243.00 a± 238.17 |
80.67 a± 89.19 |
134.40 a± 120.30 |
||
Triglycerides- M1 |
DM |
597.25 a± 479.71 |
610.33 a± 399.78 |
300.50 a± 17.67 |
|
CS |
358.40 a± 161.86 |
165.00 a± 58.00 |
303.40 a± 117.91 |
||
Triglycerides – M2 |
DM |
1127.50 a± 825.32 |
839.33 a± 299.20 |
365.00 a± 45.26 |
|
CS |
692.20 a± 256.67 |
504.00 a± 288.86 |
622.20 a± 329.83 |
||
Table 4 Means and standard deviation of decrease (initial value minus value at 30 or 60 days) in cholesterol and triglyceride levels after 30 and 60 days of continuous omega-3 administration, categorized by dose and disease
A reduction in liver enzyme activity was observed, and regardless of whether there was a greater reduction in one or other of the brands, with a statistically significant difference, in both brands the values of liver enzymes were within the reference for the species. The reduction in liver enzyme activity with a statistical difference between M0 and M2 was observed at practically all times and doses, except for FA at a dose of 75mg/kg. This result suggests that PUFAs, associated with the treatment of the primary disease and feeding, contributed to the reduction in complications associated with dyslipidemia, such as steatosis and cholestasis that promote the elevation of liver enzymes due to the use of drugs to control the primary disease and/or complications as well as a diet with reduced calories and fat, it is not possible to state that the reduction in the activity of liver enzymes is exclusively due to the use of PUFAs, however, it can be inferred that they contributed to these reductions. To date, few studies have been performed comparing biochemical results before and after supplementation with PUFAs in patients with metabolic and endocrine disorders, only in healthy patients, although there are numerous studies on hyperlipidemia and more appropriate ways to measure triglycerides and cholesterol. Steatosis of different degrees, identified by abdominal ultrasound, was identified in the 43 dogs at baseline. Of the 43, only 23 underwent ultrasound at 30days and 27 at 60days (16at 30days). Of these 27dogs there was a reduction in the degree of steatosis in 19. The reduction in steatosis observed in the ultrasound examination in 70.4% of the dogs (19/27) cannot be attributed exclusively to the control of dyslipidemia, but also to the control of endocrine and metabolic diseases, which was not analyzed in this study. In addition, the ultrasounds were performed by different professionals and using different devices in the majority of patients, which makes it difficult to analyze these results. Despite the limitations described above, it is possible to state that there was a reduction in these complications and in some cases only a decrease in enzyme activity was observed.1 ,9,12,13,19,20,27
Regardless of dose and brand, urea and creatinine levels remained unchanged during treatment. There was a decrease in FA after 60days of treatment in the groups of both brands (p>0.05), except for animals treated with 75mg/kg of brand 1, where FA levels did not demonstrate statistically significant alterations (p<0.05). A decrease in GGT was observed after 60days in the three groups of animals treated with brand 2. In animals that received brand 1, the decrease in GGT occurred only in the group treated with 75mg/kg (p<0.05). In the groups that received the doses of 100 mg/kg and 200mg/kg, GGT did not demonstrate a statistically significant change (p<0.05). There was a decrease in ALT after 60days of treatment in the groups of both brands and for almost all doses, except for animals treated with 100mg/kg of brand 1, which demonstrated unaltered ALT levels (p<0.05). The decrease observed for the cholesterol, triglyceride, urea, and FA levels was equivalent for the three doses tested and for both brands.2 with no statistical difference regarding the reduction in parameters (p<0.05).
The decrease in triglyceride levels after 30and 60days of treatment showed no difference between groups (p<0.05) for the three doses tested, both in the group of animals categorized as mild and moderate and in the group of animals classified as severe, confirming the aforementioned. Thus, the three doses had similar effects regardless of disease severity.3 Therefore, a dose of 75mg/kg of omega-3 can be used to control dyslipidemia, regardless of the severity of the disease. Dosage much lower than that currently described in the literature, which ranges from 200 to 300mg/kg (total daily dose), orally, Dose reduction will contribute to lower cost, reduction of side effects and increased adherence of owners to treatment. As mentioned earlier, a case of primary hyperlipidemia in Schnauzer was not well controlled even with higher doses of omega-3. Therefore, the results of the study suggest that PUFAs should not be the first therapeutic choice in these cases, as the delay in controlling severe hyperlipidemia may increase the risks of complications related to hyperlipidemia, such as cholestasis, mucocele, and pancreatitis.1,6,8,10,13,18,19,27
The decrease in cholesterol and triglyceride levels after 30 and 60days of treatment was equivalent (p>0.05) for the three doses tested, both in the group of animals with diabetes mellitus and in the group of animals with Cushing's syndrome (hyperadrenocorticism). Thus, the three doses had similar effects regardless of the disease. In the other diseases, this analysis was not possible due to the small number of animals in the subgroups. Therefore, the lowest dose of PUFAs was enough to contribute to the reduction in serum concentrations of triglycerides and cholesterol in dogs with Diabetes Mellitus and Cushing's Syndrome when associated with specific therapy, since dyslipidemia is an important complication in these endocrinopathies. More studies with other metabolic and endocrine diseases are needed, as well as with a greater number of animals, in order to identify how much the use of PUFAs contributes to this reduction. In a previous study in healthy dogs, a reduction in triglyceride and cholesterol levels was observed.13
In the current study, the analysis of response to treatments with different PUFA doses did not aim to correlate good control of the underlying disease with reductions in serum concentrations, as there are many factors that can influence the control of endocrine diseases such as dose and treatment time. Except in primary hyperlipidemia, where diet and PUFAs were the only treatments used, as described in the literature, all other dogs received some associated medication. At the end of the study, four dogs were still overweight, five dogs had hyperadrenocorticism, and two diabetic dogs were symptomatic, but with control of cholesterol and triglyceride levels. Therefore, both PUFAs and good control of the underlying diseases contributed to the control of dyslipidemia, even at doses of 75 and 100mg/kg.23,25,27,28
Of the 43 patients who used omega-3 for 60days, the following side effects were observed: there was an increase in flatulence in 23 dogs (8 in G1, 6 in G2, and 9 in G3), transient diarrhea in nine (2 in G1 and G2, 5 in G3), transient vomiting in four (2 in G2 and G3), transient abdominal pain in two patients in G3, and steatorrhea in one patient in G2. However, these side effects cannot be associated only with the use of PUFAs, as many of these patients were also receiving other drugs such as antibiotics, therapy for the underlying disease, among others. An excessive fishy odor was reported in 35dogs and difficulty in administering capsules in 28 dogs. Based on our study, we can conclude that the PUFA dose of 75mg/kg can be used to control dyslipidemia regardless of the cause of the disease. The main limiting factors were difficulty in administration, odor, flatulence, vomiting, and/or diarrhea. It is noteworthy that in this study, the side effects that led to the clients giving up the therapy did not occur in patients receiving the 75mg/kg dose, except in one patient when the client gave up on the treatment due to the odor of the product. Additional studies are needed to identify whether the higher doses caused side effects and/or whether underlying diseases contributed. The use of lower doses of PUFAs, when compared to those commonly recommended in the control of dyslipidemia, caused fewer side effects and minimized the cost of treatment, which can increase adherence and the feasibility of its use for the treatment of dyslipidemia. Although the study was not comparative, it apparently had fewer side effects than those described with bezafibrate in patients with different endocrine and metabolic diseases.3,7,13,14
The veterinary literature on the use of lipid-lowering drugs is scarce, and the use of fibrates for successful treatment of hyperlipidemia in dogs is based more on clinical experience and extrapolation of knowledge about their use in humans than on scientific evidence. In a study carried out in humans comparing PUFAs with bezafibrate, it was observed that both are effective in reducing triglyceride values, although bezafibrate had a more important action than PUFAs on the reduction in inflammatory cytokines. Recently, observed the safety and important efficacy of bezafibrate in dogs with primary and secondary hyperlipidemia and thus, its use may be indicated in dogs in which PUFAs and diet were not effective. It is noteworthy that in the current study, patients on fibrate discontinued use 15days before the start of the study, so that there was no influence on the results.3,11,14,27
It is known that diet is also an important factor in the control of dyslipidemia.reported that the use of pea and barley as a source of starch in diabetic dogs may help to reduce hyperlipidemia compared to the use of corn. In addition, high-fiber, lower-fat diets also seem to act in the control of dyslipidemia in diabetics. According to, in the presence of hyperlipidemia, the underlying cause should always be investigated and treated, and a switch to a low-fat diet should always be instituted, with or without omega-3 supplementation, regardless of the use of lipid-lowering drugs, which is in accordance with what was performed in the present work.2,4,13,18,19
Although the use of medication is only a recommendation when hyperlipidemia and/or dyslipidemia is severe, in the present study we chose to include patients with different degrees of dyslipidemia.18,27
The main limitations of this study were the dependence on the client adhering faithfully to the therapy administration guidelines, the inclusion of patients from different veterinary clinics, and the measurements of biological markers in different biochemical devices. In addition, the small number of patients in the different categories of metabolic or endocrine diseases was also a limiting factor. Longer studies need to be carried out, especially in patients with primary hyperlipidemia and in patients with severe dyslipidemia, to provide evidence of the long-term efficacy in these cases. In the present study, a longer follow-up and performance of other tests would be quite enriching, but were not feasible due to the costs. An analysis of the omega-3 capsules was also carried out to identify whether the concentrations present on the labels were correct.
It is concluded that the 75mg/kg dose of PUFAs decreases the severity and/or controls dyslipidemia in different metabolic and endocrine diseases. It is concluded that PUFAs are safe for use in patients with primary hyperlipidemia and endocrine disorders. It is concluded that a dose of 75mg/kg PUFAs was sufficient to decrease triglycerides and cholesterol in dogs with Diabetes Mellitus and Cushing's Syndrome when associated with specific therapy.
None.
The author declares there are no conflicts of interest.
None.

©2022 Cardoso, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.