Journal of
eISSN: 2377-4312


Research Article Volume 10 Issue 1
Pathology Department, Faculty of Veterinary Medicine, Mansoura University, Egypt
Correspondence: Walaa F Awadin, Pathology Department, Faculty of Veterinary Medicine, Mansoura University, Egypt
Received: February 11, 2021 | Published: March 30, 2021
Citation: Awadin WF, Elshaieb AF, EL-Morshidy Y. Retrospective pathological studies on gastrointestinal tract parasitic diseases in cattle and buffaloes at EL- Dakahlyia province. J Dairy Vet Anim Res. 2021;10(1):5-9 DOI: 10.15406/jdvar.2021.10.00304
Background and objective: The present study was carried out to study gross pathology, histopathological examination of gastrointestinal tract (GIT) of animals affected with helminthes and protozoan parasites in cattle and buffaloes slaughtered in Mansoura governmental abattoir and necropsied in local dairy farms at EL-Dakahlyia Province.
Materials and methods: Different kinds of trematodes, cestodes and nematodes were generally screened during the period 2017-2020 by post-mortem examination of 12,250 slaughtered bovine carcasses.
Results: Out of 12,250 slaughtered bovine carcasses, 3604 cases (29%) were affected with gastrointestinal parasites. Helminthes and protozoan parasites were encountered in 3209 (89%) and 395 (10.9%) cases, respectively. The observed helminthes were nematodes (Gongylonema pulchrum, toxocara vitillorum), trematodes (Fasciola; F. gigantica) and cestodes (Moniezia, hydatid cyst), while sarcosporidia, eimeria and cryptosporidia were the encountered protozoa. Grossly, fasciola and hydatid cysts were detected in liver, gongelonema pulchrum, major sarcocysts were detected in esophagus, toxocara vitillorum were detected in small intestine. Minor sarcocysts, intestinal cryptosporidiosis and coccidiosis were discovered microscopically. The histopathological lesions were graded from 0 (no lesion), I (mild), II (moderate) to III (severe).
Conclusion: No or minimal lesions as observed with living gongelonema, major and minor sarcocysts to severe caseation, calcification and granuloma formation as demonstrated with hydatid cysts and fasciola.
Keywords: parasitic infestation, git, cattle, gross, histopathology
The gastrointestinal tract (GIT) of animals harbor different kinds of parasites particularly helminthes, which adversely affect productive and reproductive performance of the cattle.1 Loss of productivity resulted from reduction of body weight due to long period of digestive disturbance.2 Almost mature worms produce toxins that destroy red blood cells, leading to unthrifty anaemic condition.3 Immature worms migrating through the body tissues open the way for bacteria and fungi to enter, causing some other serious diseases.3 Other economic losses were poor work performance, involuntary culling, lower milk production, abortion, infertility, treatment costs, and mortality in heavily parasitized animals.4–6 Among the predisposing factors of internal parasites infection are climates, nutritional deficiency, grazing habits, immunological status, pasture management, presence of intermediate host and vector and the number of infective larvae and eggs in the environment.2 Other factors such as breed, age, environment, ecology and pathogenicity of the parasites can influence the occurrence of gastrointestinal parasitism.7 In the intestinal tract, these parasites lay minute eggs that are found in the animal’s feces. The eggs undergo development into larvae. Within some days especially during warmer period, further development of the larvae to an infective stage occurs. While on the ground, the larvae can migrate from the fecal sample to other grasses close by or the animals can step on their dung; thereby enhancing further spread of the larvae. The animals however get infected through the ingestion of the contaminated grass.8 Knowledge on prevalence of these parasites is mandatory. Unfortunately, little or no clinical signs appeared on infected animals with parasites.9,10 So abattoir survey considered an excellent way of determining the prevalence of ruminant parasitic diseases as to control the disease through regular de-worming, proper feeding and good sanitary measures.8 The present research was designed to record parasitic profile of GIT in slaughtered and necropsied cattle and buffaloes to give awareness to the farmers and veterinarians about the parasitic infestation causing severe tissue damage in our locality to suggest proper treatment, control and preventive measures.
Animals & tissue specimens
A total of 12,250 bovine carcasses (cattle & buffaloes) of both sexes and different ages were slaughtered in Mansoura governmental abattoir and necropsied in local dairy farms at EL-Dakahlyia Province during the period March to January 2017-2020. Examination of the GIT began with a thorough examination of the oral cavity (including lips, gums, hard and soft palate, teeth, and tongue), esophagus, fore-stomachs and true stomach (abomasum), small and large intestines, liver and pancreas to find out internal parasitic infestation and any gross pathological changes. Specimens were taken from the parasites and surrounding tissue and fixed in 10 % neutralized formaldehyde. The rate of infection among slaughtered cattle obtained from the percentage of infected samples to total examined cases. The fixed samples were routinely processed in ascending grades of alcohol, xylol, embedded in paraffin wax, sectioned at 4–5μm, and stained with hematoxylin and eosin (HE) for histopathologic examination. The prepared sections were examined under light microscope. Microscopic lesions caused by helminthes and protozoan parasites were graded into no lesion (grade 0), a mild infection characterized by simple or mild in their nature with very good reversible prognosis (grade I), moderate infection characterized by moderate severity of the lesions with a good reversible prognosis (grade II) and severe infection characterized by hostile severity with bad irreversible prognosis as a result of architecture changes in tissue histology (grade III). The protocol for examination of histopathological slides was previously described by Corley et al.11
Out of 12,250 slaughtered and necropsied bovine carcasses, 3604 cases (29%) were affected with gastrointestinal parasites. Helminthes were encountered in 3209 (89.15%) cases. The numbers of carcasses affected with trematodes (Fasciola, F. gigantica) were 358, nematodes (Gongylonema pulchrum, toxocara vitillorum), were 1828, cestodes (Moniezia, hydatid cyst) were 23. While, the protozoan parasites (sarcosporidia, eimeria and cryptosporidia) were encountered in 395 (10.9%) cases.
Trematodes
Gross lesion of liver affected with chronic fascioliasis showed enlargement in size (hepatomegaly) and pinpoint hemorrhages on the parietal surface of the liver, paleness in some areas which was due to the necrotic or damaged region, congestion, firm whitish areas that regarded as fibrosis. Wall of the affected bile duct was thickened hard to cut. Lumens of bile ducts were engorged with bile or filled with blackish brown exudate or blocked by twisted flukes. The histopathological changes associated with presence of adult F. gigantica were characterized by biliary hyperplasia (Figure 1a), necrosis (Figure 1b) and calcification (Figure 1c), portal fibrosis associated parenchyma inflammation, degeneration, necrosis (Figure 1d). Biliary necrosis (Figure 1e), calcification and periductal fibrosis were observed in bile ducts occluded with laid eggs (Figure 1f). According to severity of lesion it was graded III.
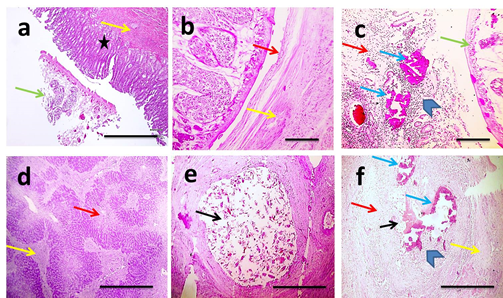
Figure 1 Microscopic pictures of H&E stained hepatic sections showing adult F. gigantica in lumen of large bile duct (green arrow) with thickened wall due to adenomatous hyperplasia of biliary epithelium (asterisk) and fibrosis (yellow arrow) (a), necrotic duct wall (green arrow) surrounded by fibrous tissue deposition (yellow arrow) (b), foci of basophilic calcification (blue arrow) appears in case of necrotic duct wall surrounded by mixed inflammatory cells (arrowheads) (c). Multifocal areas of coagulative necrosis (red arrow) and fibrosis (yellow arrow) (d). Lumen of bile duct expanded with laid eggs of F. gigantica (black arrow) (e). Wall of bile duct contained eggs of F. gigantica (black arrow) is necrotic (red arrow), inflamed (arrowhead), calcified (blue arrow) and surrounded by fibrous tissue (yellow arrow) (f). X:40 bar 200 (a,d,e,f) and X:100 bar 100 (b&c).
Nematodes
Gross appearance of gongylonema pulchrum was pale white to orange red spirurid parasites burrowed in esophageal upper mucosa (Figure 2a). Microscopically, the parasites were observed in stratified squamous epithelial lining without histopathological lesions (Figure 2b). According to severity of lesion, it was graded 0. The gross lesions in the small intestine affected with adult round worms were focally thickening of intestinal wall with hyperemic mucosa. The intestinal lumen was obstructed by these worms. Histopathological examination revealed presence of adult female worm in intestinal lumen and its deposited eggs associated with diffuse coagulative necrosis of intestinal villi or multifocal loss of villi and the remaining villi were blunted and fused with erosion of the mucosa (Figure 2c). Intestinal crypts were dilated necrotic cellular debris and degenerate inflammatory cells (crypt abscesses) overlying hyperplastic Peyer's patches (Figure 2d). Severity of lesion due to toxocara vitulorum was graded II.
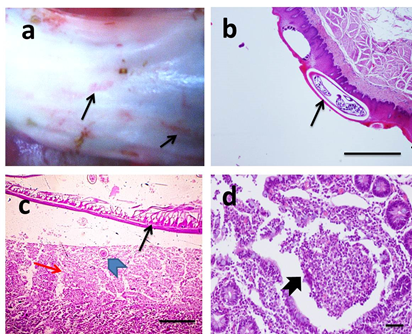
Figure 2 Gross picture of opened esophagus.
Showing presence of red spirurid gongolonema burrowed in esophageal mucosa (black arrow) (a). Microscopic pictures of H&E stained esophageal sections showing different stages of nematode worms surrounded by a clear space in upper epithelial lining (black arrow) without tissue reaction (b). Microscopic pictures of H&E stained intestinal sections showing adult female toxocara worm in lumen (thin black arrow) and its deposited egg (arrowhead) in enteric mucosa associated with coagulative necrosis of intestinal villi (red arrow) (c). Crypt abscess (thick black arrow) is observed in other intestinal sections accompanied ascariasis (d). X:40 bar 200 (b), X:100 bar 100 (c) and X:400 bar 50 (d).
Cestodes
The anoplocephalid tapeworms (Moniezia spp.) were detected attached to intestinal mucosa. The site of attachment on the intestinal mucosa may be indicated by the presence of a small ulcer and a mild inflammatory response (Figure 3a). Histopathological examination of intestinal mucosa revealed focal ulceration, mild leukocytic cells infiltration in lamina propria and submucosa associated with dilated blood vessels (Figure 3b). Severity of lesion due to tapeworms was graded I.Gross appearance of hydatid cysts revealed single to multiple cysts of varying size were observed from the visceral and/or parietal surfaces of liver (Figure 4a). In general the cysts were soft and doughy to touch and were filled with clear to slightly turbid fluid.The incised cysts contained a turbid, amber liquid with hydatid sand. However, some cysts were appearing firm and contained inspissated contents. Also, some cysts were calcified, gritty and hard to cut. Microscopically, hydatid cysts may be living (sterile&fertile) and dead. Histopathological examination of sterile cysts displayed three-layered wall architecture, with an outer, host-derived adventitial layer, an acellular intermediate laminated layer, and an inner germinal membrane of parasitic origin (Figure 4b–4d). The outer adventitial layer consisted of a chronically inflamed fibrous tissue with scattered foci of calcification. In fertile hydatid cyst, daughter cyst showing broad capsule containing many scolices was seen in some cases (Figure 1e). Lesions detected in adjacent hepatic tissue were hydropic degeneration, focal steatosis, necrosis, aggregations of macrophages and Langhan's giant cells (Figure 1f), expanded portal areas with fibrosis (Figure 1g), dilated blood vessels and inflammation,. Meanwhile, histopathological examination of dead cysts displayed necrotic hyalinized germinal membrane surrounded by thick fibrous wall. In those cases, lesions detected in adjacent hepatic tissue were telangiectasia, mild degeneration and few leukocytic cells infiltration (Figure 4h). Severity of lesions due to hydatid disease was graded from I (mild) in case of dead cysts to III (severe) in case of living cysts.
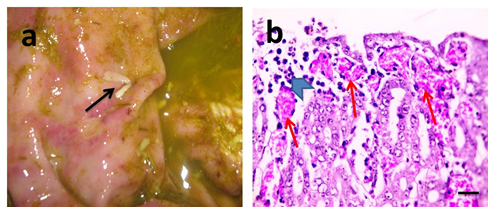
Figure 3 Gross picture of injured intestinal mucosa.
Showing tapeworms attached to mucosa. Their segments are wider than they are long (black arrows) (a). Microscopic pictures of H&E stained intestinal sections showing mild mixed leukocytic cells infiltration (arrowhead) and dilated blood vessels in lamina propria (red arrows) (b). X:400 bar 50 (b).
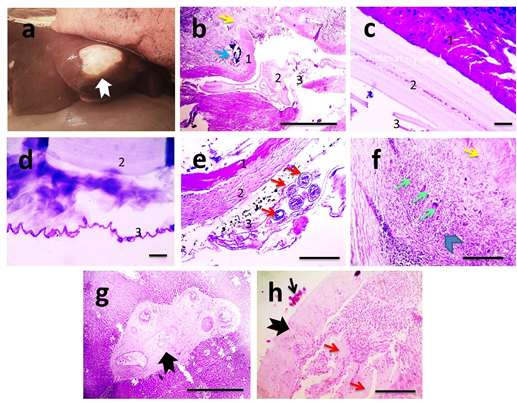
Figure 4 Gross picture of liver.
Hydatid cyst with thickened opaque wall (black arrow). Their segments are wider than they are long (a). Microscopic pictures of H&E stained hepatic sections showing three-layered wall architecture, with an outer, host-derived adventitial layer (1), an acellular intermediate laminated layer (1), and an inner germinal membrane of parasitic origin (3). The outer adventitial layer consisted of a chronically inflamed fibrous tissue (yellow arrow) and scattered foci of calcification (blue arrow) (b). Higher magnifications of cyst wall appear in (c&d). In fertile hydatid cyst, daughter cyst showing broad capsule containing many scolices (red arrows) is seen (e). Adjacent hepatic tissue shows granuloma (arrowhead) consisted of aggregation of lymphocytes, macrophages and Langhan's giant cells (green arrows) surrounded by fibrous tissue (yellow arrow) (f), marked portal fibrosis (thick black arrow) (g). In case of dead hydatid cyst, necrotic hyalinized germinal membrane is seen (thin black arrow) surrounded by thick fibrous wall (thick black arrow). Adjacent hepatic parenchyma displays telangiectasia (red arrows) (h). X:100 bar 100 (b,e,f) X:400 bar 50 (c,d,h).
Protozoan parasites
Coccidiosis and cryptosporidiosis was diagnosed microscopically because non-specific gross lesions such as thickened, congested and edematous intestinal mucosa particularly of the ileum and large intestine were observed. In case of coccidiosis, microscopic examination of the intestinal mucosa showed congested blood vessels and the lamina propria was moderately infiltrated with mixed inflammatory cells composed mainly of neutrophils, few lymphocytes, plasma cells, and macrophages that extended to muscular coat. Multifocally, the lining epithelium of intestinal villi and crypts of Lieberkuihn were markedly distorted by abundant intracellular eimeria in varying stages of development in the enterocytes (Figure 5a). The glandular epithelium was extremely attenuated, and disrupted with releasing of developmental stages and oocytes into the lumen of glands. There is also segmental enterocyte necrosis with villous blunting in several sections of small intestine. Occasionally, crypts abscesses were detected. In some sections coccidian oocytes were seen in the lumen of the small intestine among sloughed epithelial cells, necrotic debris and infiltrates of inflammatory cells mainly neutrophils with fewer lymphocytes and macrophages. Rarely erythrocytes were observed in the lumen admixed with the desquamated epithelium. The submucosa was expanded by edema admixed with moderate number of inflammatory cells, with dilated lymphatics and congested blood vessels. According to severity of lesion it was graded II. In case of cryptosporidiosis, microscopic examination of the intestinal mucosa exhibited moderate villous atrophy characterized by blunting and fusion of villi; meanwhile crypts of Lieberku¨hn revealed hyperplasia and hypertrophy of lining epithelium. Crypts abscesses were occasionally seen. Variable numbers of amphophilic to basophilic; round cryptosporidia protozoa were seen in the microvillus border of cells on the villi (Figure 5b). Multifocally, the lamina propria and submucosa were expanded by moderate numbers of lymphocytes, plasma cells and neutrophils and showed congested blood vessels. According to severity of lesion it was graded I. The esophagus of affected buffaloes infected with numerous macroscopic sarcocysts was distributed mainly under the serosal membrane and in muscular coat. Microscopically, major macrocysts consisted of bradyzoites tended to be overcrowded at the periphery of the cyst and decrease in number towards the center (Figure 5c). Minor sarcocysts were also observed in other muscle fibers (Figure 5d). According to severity of lesion it was graded 0.
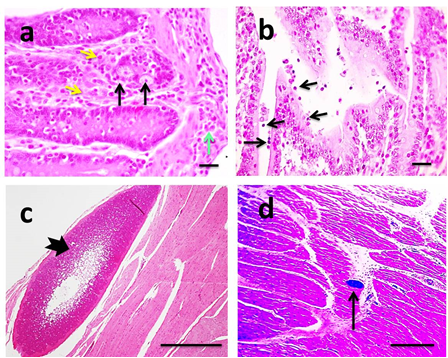
Figure 5 Microscopic pictures of H&E stained intestinal sections.
Showing eimeria gamonts in crypt epithelium (black arrows) with mixed leukocytic infiltertion in lamina propria (yellow arrow) extending to muscular coat (green arrow) (a). Moderate villous atrophy associated with presence of small many basophilic rounded bodies attached to epithelium (black arrows) (b). Microscopic pictures of H&E stained esophageal sections showing major (thick black arrow) (c) and minro (thin black arrow) (d) sarcocyst in muscle fibers. X:400 bar 50 (a&b), X:40 bar 200 (c), X:100 bar 100 (d).
Out of 12,250 slaughtered bovine carcasses, 3604 cases (29%) were affected with gastrointestinal helminthes and protozoan parasites in 3209 (89%) and 395 (10.9%) cases, respectively. The observed helminthes were nematodes (Gongylonema pulchrum, toxocara vitillorum), trematodes (Fasciola; F. gigantica) and cestodes (Moniezia, hydatid cyst), while sarcosporidia, eimeria and cryptosporidia were the encountered protozoa. Ruminants are commonly infected by nematodes such as Strongyliodes species, Trichuris species, Ostertagia species, Capillaria species; cestodes such as Taenia species, Moniezia species and trematodes such as F. gigantica, Dicrocoelium species.12 Microscopic results of chronic fascioliasis was previously reported by results showed that the most affected sites were portal area especially bile ducts, where the inflammatory reaction started then extended to other parts of hepatic tissue.13 Biliary hyperplasia, necrosis and calcification, portal fibrosis associated parenchyma inflammation, degeneration, necrosis, were the main microscopic findings that scored III as mentioned by Al-Mahmood and Al-Sabaawy.14 Microscopic examination of intestine infected with ascariasis showed disorganized villous structures due to sever mucosal epithelium desquamation. The mucosal and submucosal blood vessels were engorged with blood. The lamina propria was infiltrated with neutrophils and few eosinophils along with small areas of hemorrhages as mentioned by Hassan et al.15 Hydatid disease is an infection of herbivores by cyst of Echinococcus granulosus.
When mature hydatid cyst having numerous protoscoleces ingested by a carnivore such as dog, it develops into an adult worm.16–18 Hydatid cyst is typically filled with a clear fluid (hydatid fluid) which is bacteriologically sterile.19 In the present study the cyst wall in liver presented the characteristic trilaminar structure with germinal membranes and brood capsules, and free scolex as reported earlier by.20–22 Adjacent hepatic areas to the cyst wall showed congestion, haemorrhage, hepatocyte necrosis fibrosis and inflammation as reported by.23 Fibrosis seen in the portal area was also reported by.22 Further, the extensive fibroplasia and cirrhosis observed in some cases was attributed to immunological reactions of the host tissue.24 Proliferative and degenerative changes in biliary epithelium were also reported by Solcan et al.25 in some affected livers. Gross and histopathological examinations constitute a golden tool for postmortem diagnosis of hydatidosis.26 The anoplocephalid tapeworms (Moniezia spp) are characterized by the absence of a rostellum and hooks, and the segments are wider than they are long. Infection occurs after ingestion of infected mites. However, Moniezia was considered nonpathogenic, young calves harbored several of these worms can be affected with troubles in digestion and poor growth.27 Microscopic examination of intestinal mucosa infected with cryptosporidiosis exhibited moderate villous atrophy stunting and fusion of villi as stated by.28–30 Hyperplasia and hypertrophy of epithelial lining crypts of Lieberkuihn was also reported by.30,31 Thus, immature regenerative cells replaced damaged enterocytes.30 Moreover, increased cellularity in lamina propria with marked eosinophilia was focally demonstrated in distal jejunum, ileum and caecum.29
In current study, cellular infiltration was consisted of moderate numbers of lymphocytes, plasma cells and neutrophils. Variable numbers of amphophilic to basophilic; round cryptosporidium protozoa were seen in the microvillus border of cells on the villi. Microscopic examination of intestinal mucosa infected with coccidiosis revealed presence of abundant variable intracellular developmental stages of Eimeria in lining epithelium of crypts. Microscopically, the blood vessels in the mucosa were hyperemic as described by.32,33 According to Daugschis and Najdrowski34 the enormous reproduction of the parasite during sexual multiplication induced the extensive destruction of the intestinal mucosa. Crypts were expanded by necrotic cellular debris and degenerate inflammatory cells (crypt abscesses).35,36 The lamina propria was moderately infiltrated with mixed inflammatory cells composed mainly of neutrophils, few lymphocytes, plasma cells, and macrophages. These findings were closely similar to those mentioned by.32,33 Villous blunting was seen in several sections of small intestine as observed by Sameer et al. 36 in calves slaughtered at 25 day post infection. Furthermore, macroscopic sarcocysts were exclusively found in the esophagus.37,38 In Egypt, Sarcocystis spp. infection was still of low prevalence.39 Inflammation and necrosis were not observed in affected esophagus. However, macroscopic sarcocysts in meat may be considered an economic and sanitation problem due to condemnation of infected carcasses.40
The histopathological lesions were graded from no or minimal lesions as observed with living gongelonema, major and minor sarcocysts, moderate in coccidioisis, cryptosporidiosis and severe in hydatidosis and fasciolasis.
This study showed that gross and histopathological examinations of large ruminants through periodical abattoir survey and necropsy give a good idea about most common and severe parasitic diseases that required regular de-worming, proper treatment, control and preventive measures.
None.
The author declares no conflict of interest exists.
None.

©2021 Awadin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.