Journal of
eISSN: 2377-4312


Research Article Volume 9 Issue 2
1Faculty of Veterinary Medicine, Shahrekord University, Iran
2Department of Pathobiology, Faculty of Veterinary Medicine, Shahrekord University, Iran
3Department of Clinical Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Iran
4Department of Clinical Sciences, Faculty of Veterinary Medicine, Shahrekord University, Iran
Correspondence: Amir Dehghani-Samani, Faculty of Medicine, Birjand University of Medical Sciences and Health Services, Birjand, Iran
Received: February 17, 2019 | Published: March 20, 2020
Citation: Dehghani-Samani A, Pirali Y, Madreseh-Ghahfarokhi S, et al. Parasitic infection status of different native species of Columbidae family in southwest of Iran. J Dairy Vet Anim Res. 2020;9(2):45-51. DOI: 10.15406/jdvar.2020.09.0027
In current investigation, for the first time, prevalence of parasites in different native members of columbidae family were studied carefully in Shahrekord, located in southwest of Iran. Totally 220 birds from 4 different species were examined for presence of every ectoparasites and endoparasites by use of identification keys for parasites. After preparation of blood smears, oral cavity and crop wet smears, feces samples and their direct smears, flotation of feces and evisceration of examined birds, isolation and identification of parasites were done via laboratory methods and identification keys. Results of current study show the occurrence and prevalence of different parasites in examined groups. The common blood parasite with highest prevalence is Haemoproteous columbae in Rock pigeons (27.27%). The highest prevalence of Leukocytozoon marchouxi is for Rock Pigeons (5.54%). Columbicola columbae is the common ectoparsite with highest prevalence in Rock pigeons (56.36%), the highest prevalence of Menopon gallinae is for Rock Pigeons (21.81%), also the highest prevalence of Pseudolynchia canariensis and Lipeurus caponis are for Rock pigeons (36.36% and 16.36% respectively). The highest prevalence of Trichomonas gallinae is for Rock pigeons (67.27%). The highest prevalence of Eimeria labbeana is for Laughing Doves (30.90%). Highest prevalence of Cryptosporidium meleagridis is for Rock pigeons (3.63%) and the highest prevalence Echinostoma revolutum is for Rock Pigeons (20%). Ascaridia columbae has the highest prevalence in Domestic Pigeons (25.45%). The highest prevalence of Ascaridia galli, Raillietina echinobothrida and Raillietina tetragona are for Rock Pigeons (7.27%, 18.18% and 12.72% respectively). Results of current study for the first time show the considerable level of parasitic infections in the examined groups in this area.
Keywords: Columbidae family, ectoparasite, endoparasites, iran, Shahrekord
Doves and pigeons are different genus of columbidae family, Columbiformes order.1 They are ubiquitous birds and can be found in virtually every town and city around the globe.2 Pigeons are related to human since ancient time, they live side by side with human as a source of food, hobby and experimental purposes.3 Parasites are regarded as the basic causes of retardation in growth, lowered vitality and poor conditions of the birds. They can affect bird's health directly by causing irritation, discomfort, tissue damage, blood loss, toxicosis, allergies and dermatitis which in turn reduce the quality and quantity of meat and egg production.4
The domestic pigeon (Columba livia domestica) is a pigeon that was derived from the rock pigeon (Columba livia). The rock pigeon is the world's oldest domesticated bird. Mesopotamian cuneiform tablets mention the domestication of pigeons more than 5,000 years ago, as do Egyptian hieroglyphics. Research suggests that domestication of pigeons occurred as early as 10,000 years ago.5 The laughing dove (Spilopelia senegalensis) is a small pigeon that is a resident breeder in Sub-Saharan Africa, the Middle East area to the Indian Subcontinent. This small long-tailed dove is found in dry scrub and semi-desert habitats where pairs can often be seen feeding on the ground.6 The Eurasian collared dove (Streptopelia decaocto) that often simply called the collared dove is a species of dove native to warm temperate and subtropical Asia. It is a medium-sized dove, distinctly smaller than the wood pigeon, similar in length to a rock pigeon but slimmer and longer-tailed, and slightly larger than the related turtle dove, it is not migratory, but is strongly dispersive. Over the last century, it has been one of the great colonies of the bird world, they typically breed close to human habitation wherever food resources are abundant and there are trees for nesting.7
Ectoparasites are organisms which inhabit the skin or outgrowths of the skin of another organism (the host) for various periods, and may be detrimental to the latter. Various ectoparasites cause significant infestations in many kinds of domestic animals including livestock, pets, laboratory animals, poultry, fish and bees. Many of these ectoparasites (e.g. most lice) are host specific, while others (e.g. many ticks) parasitize a wider range of hosts.8 Endoparasites are organisms that, in their developmental or adult stages, live in animals called hosts. Endoparasites, which include single-celled protozoa, worms (helminths), and arthropods, invade nearly all organs of animals. Protozoans are found in digestive and respiratory systems, muscles, blood, and feces of their hosts. Several endoparasitic worms feed on the ingesta in the intestine of the definitive (final) host or are attached to the mucosal layer within the intestine or the trachea where they suck blood or epithelial cells. Other worms are found in specific organs or only parts of organs. Parasitic arthropods, including ticks, mites, flies, mallophages, and fleas, often are found on the skin or feathers of their hosts. Only a few arthropods enter the internal organs of the hosts. Endoparasitic arthropods include mites living in layers of the skin or subcutaneously, and the larval stages of flies (maggots) that burrow through internal organs.8,9
There are so many flocks of pigeons and doves in Shahrekord, southwest of Iran, and peoples are related to these species, pigeons and doves are found anywhere and contact with humans and other birds, some peoples feed from their meat as a favorite source of digestible proteins. Pigeons farming done for very different purposes such as: use of their meat, flying, use of their eggs and use for beautiful pets in houses and near the humans. To the best of authors knowledge there is no study on prevalence of parasites in resident native members of Columbidae family in Shahrekord, southwest of Iran. Because of the risk of zoonosis diseases that may carry by pigeons and transition of non-host specific parasites to other birds, in this study we tried to introduce the common parasites of pigeons and doves in this area and find the prevalence of ectoparasites and endoparasites of pigeons and doves in Shahrekord, southwest of Iran.
Study area and sampling data
This study located in Shahrekord (latitude, 32°19' 32" N and longitude, 50°51'52" E) in southwest of Iran. 55 Domestic Pigeons (Columba livia domestica), 55 Rock Pigeons (Columba livia), 55 Laughing Dove (Spilopelia senegalensis) and 55 Eurasian Collared Dove (Streptopelia decaocto) collected from different parts of Shahrekord in Charmahal va Bakhtiari province in Iran (Figure 1).

Figure 1 Examined Birds: A pair of Domestic Pigeons (Columba livia domestica) examined in study (a), Rock Pigeons (Columba livia) with grey color in most of birds (b), Laughing Dove (Spilopelia senegalensis) with light/dark brown color (c) and Eurasian Collared Dove (Streptopelia decaocto) with light grey color and collar in neck (d).
Examination and identification of Blood parasites
A small amount of blood (~50μl) from brachial vein via sterile vein puncture was taken. Immediately blood smears prepared and were air dried and fixed in absolute methanol for 5 minutes after sample collection and later stained with Wright-Giemsa, staining techniques for 15 minutes.10 After the staining and drying of smears, the slides were observed by optic microscope (Via lens 10, 40 & 100) carefully for identifying of blood parasites. Blood parasites identified in accordance with the keys of identification.11,12 Number of collected ectoparasites and infested birds recorded carefully.
Examination and identification of ectoparasites
The ectoparasites contains louses, mites, ticks, flies and fleas collected from surface of skin, feathers, around the vent, under the wings and back of heads (necks) of infested birds. Collected ectoparasites were transferred to vials containing 70% ethanol alcohol and stored in the laboratory until microscopic examination for identification. The number of collected ectoparasites and infested birds recorded carefully. In laboratory, the ectoparasites were fixed in a 75 % filtered ethanol solution (72 hours), cleared in 85 % lactic acid (24 hours) and mounted separately on slides using Hoyer fluid after being cleared in lacto-phenol and identified by light microscope (Via lens 4, 10 & 40) in accordance with the keys developed by Clay,13,14 Price and Beer,15 Clayton,16 Martin-Mateo17 and Adams et al.18
Examination and identification of gastro-intestinal tract's parasites
At the first gross examination of oral cavity was done and swab was taken from the throat or from the oral cavity of each bird. The swabs were processed through direct smear method and then subsequently with Wright-Giemsa, staining techniques10 to identify the Trichomonas gallinae. After the staining, smears were observed carefully by optic microscope (Via lens 10, 40 & 100) to find the occurrence and prevalence of Trichomonas gallinae according to the keys of identification,11 that Trichomonas gallinae is a single-celled, pear-shaped protozoan with 4 whip-like anterior flagella and a fin-like undulating membrane that extends for approximately 1/2 of the total body.
In next step the fresh feces samples were taken from every bird's intestinal contents via their rectum. Then three different types of qualitative tests; namely direct smear, flotation and sedimentation techniques were used to examine the fecal samples to identify the morphological features of eggs and oocystes of parasites.12 At first fecal samples washed with normal saline after centrifuged (3000 rpm for 5 minutes) and removing of upper suspension for three times, then the sediment from each sample was mixed with Sheather’s saturated sugar solution, centrifuged (3000 rpm for 10 minutes) and then examined under a optic microscope for the presence of protozoan oocysts. Coccidian species were identified according to the size and morphological characteristics of the oocysts including: shape and colour of the oocysts; thickness of the oocyst walls; presence of micropyle, cap, polar granules, oocyst or sporocyst deposits; size and shape of the sporocysts; shape of Stieda bodies and of sporozoites; etc. Also helminth eggs were also detected and identified according to their morphological features.11 In addition all the fecal samples were examined for Cryptosporidium oocysts by the modified acid-fast staining method.19
The evisceration process include complete separation of digestive tract from esophagus to vent was investigated of any presence of ant type of worms, when it found, the worms cleaned in saline and the identification done by dissecting microscope then preserved in 70% ethanol.11 For this purpose, after the slaughtering of birds, the alimentary tract was separated out and then differentiated into oesophagus, crop, proventriculus, gizard, duodenum, small intestine, caecum and rectum. Then the separated parts were put in different petri-dishes with normal saline (0.9% NaCl) and organs were opened by incision. Then helminthes that removed from gastro-intestinal tracts were fixed in Acetic Formal Alcohol and cleared in lacto-phenol solution and stained in borax carmine (diluted with lacto-phenol). Dehydration was done by a upgrading of ethanol. Collected parasites were identified according to the figure and description given by Cheng.20
Statistical analysis
Prevalence of any parasites calculated by division of number of infested/infected birds to total number of examined birds. The data were expressed as the Mean±standard error of the mean (SEM) using Sigma stat (version 3.1) software. Groups were compared using one-way ANOVA for repeated measurements. A value of (P≤0.05) was considered significant.
Identification and prevalence of blood parasites
Examination of birds for blood parasites shows that there are infections by two species of blood parasites in examined groups: Haemoproteous columbae and Leukocytozoon marchouxi (Figure 2). Occurrence and prevalence of identified blood parasites of each group are shown in Table 1. There is significant differences between the prevalence of Haemoproteous columbae and Leukocytozoon marchouxi in examined groups, the highest prevalence of Haemoproteous columbae is for Rock pigeons and the lowest prevalence of Haemoproteous columbae is for Domestic Pigeons, also the highest prevalence of Leukocytozoon marchouxi is for Rock pigeons and the lowest prevalence of Leukocytozoon marchouxi is for Domestic pigeons (Figure 3).
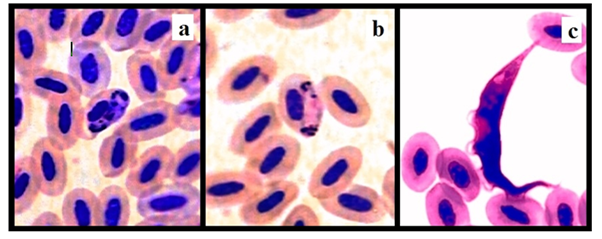
Figure 2 Identified blood parasites: Macro-gametocyte of Haemoproteous columbae in the infected erythrocytes shown in center of picture (a), micro-gametocyte of Haemoproteous columbae in the infected erythrocytes shown in center of picture (b) and Leukocytozoon marchouxi with conical shape shown among erythrocytes (c).
|
Haemoproteous columbae |
Leukocytozoon marchouxi |
|
|
Domestic Pigeons |
10 Infected/55 Birds |
1 Infected/55 Birds |
|
Columba livia domestica |
18.18% a |
1.81% a |
|
Rock Pigeons |
14 Infected/55 Birds |
3 Infected/55 Birds |
|
Columba livia |
27.27% b |
5.45% b |
|
Laughing Dove |
13 Infected/55 Birds |
0 Infected/55 Birds |
|
Spilopelia senegalensis |
23.63% b |
0.00% c |
|
Eurasian Collared Dove |
11 Infected/55 Birds |
0 Infected/55 Birds |
|
Streptopelia decaocto |
20.00% c |
0.00% c |
Table 1 Occurrence and prevalence of blood parasites in different groups were shown. Different lower cases (a–d) show the significant difference between rows of every column. Values of (P≤0.05) were considered significant

Figure 3 Identified blood parasites: the highest and lowest prevalence of each blood parasites were shown.
Identification and prevalence of ectoparasites
After the examination of birds for presence of ectoparasites, different arthropods were identified as Menopon gallinae, Pseudolynchia canariensis, Columbicola columbae and Lipeurus caponis according to identification's keys (Figure 4). The most common ectoparasite in birds was Columbicola columbae. There is significant difference (P≤0.05) between prevalence of identified ectoparasites in different groups. Occurrences of removed ectoparasites and prevalence of each of them in every group are listed in Table 2. The highest prevalence of Menopon gallinae is for Rock pigeons and the lowest prevalence of Menopon gallinae is for Laughing Doves, the highest prevalence of Pseudolynchia canariensis is for Rock pigeons and the lowest prevalence of Pseudolynchia canariensis is for Laughing Doves, the highest prevalence of Columbicola columbae is for Rock pigeons and the lowest prevalence of Columbicola columbae is for Eurasian collared Doves, the highest prevalence of Lipeurus caponis is for Rock pigeons and the lowest prevalence of Lipeurus caponis is for Laughing Doves (Figure 5).
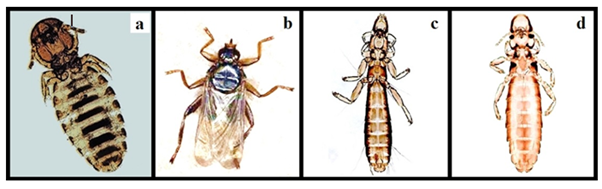
Figure 4 Identified ectoparasites: Menopon gallinae (a), Pseudolynchia canariensis (b), Columbicola columbae (c) and Lipeurus caponis (d).
|
Menopon gallinae |
Pseudolynchia canariensis |
Columbicola columbae |
Lipeurus caponis |
|
|
Domestic Pigeons |
11 Infested/55 Birds |
17 Infested/55 Birds |
23 Infested/55 Birds |
4 Infested/55 Birds |
|
Columba livia domestica |
20.00% a |
30.90% a |
41.81% a |
7.27% a |
|
Rock Pigeons |
12 Infested/55 Birds |
20 Infested/55 Birds |
31 Infested/55 Birds |
9 Infested/55 Birds |
|
Columba livia |
21.81% a |
36.36% b |
56.36% b |
16.36% b |
|
Laughing Dove |
7 Infested/55 Birds |
5 Infested/55 Birds |
13 Infested/55 Birds |
1 Infested/55 Birds |
|
Spilopelia senegalensis |
12.72% b |
9.09% c |
23.63% c |
1.81% c |
|
Eurasian Collared Dove |
10 Infected/55 Birds |
9 Infected/55 Birds |
11 Infected/55 Birds |
3 Infected/55 Birds |
|
Streptopelia decaocto |
18.18% a |
16.36% d |
20.00% d |
5.45% a |
Table 2 Occurrence and prevalence of ectoparasites in different groups were shown. Different lower cases (a–d) show the significant difference between rows of every column. Values of (P≤0.05) were considered significant
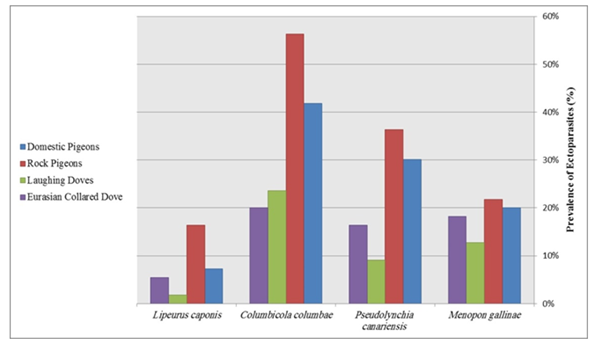
Figure 5 Identified ectoparasites: the highest and lowest prevalence of each ectoparasite were shown.
Identification and prevalence of endoparasites
There is a high prevalence of infection by Trichomonas gallinae (Figure 6a) in population of domestic pigeons and rock pigeons. There is significant difference between prevalence of Trichomonas gallinae in different groups and comparative data are shown in Table 3. The highest prevalence of Trichomonas gallinae is for Rock pigeons and the lowest prevalence of Trichomonas gallinae is for Laughing Doves (Figure 7).
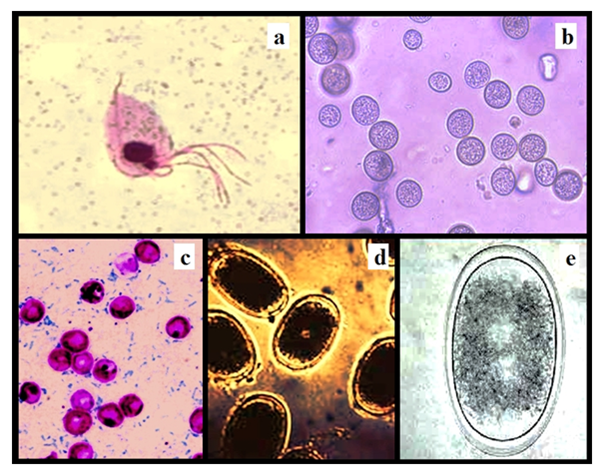
Figure 6 Identified protozoan, oocysts and egg of helminths: Trichomonas gallinae (a), Eimeria labbeana Oocysts (b), Cryptosporidium meleagridis Oocysts (c), Ascaridia columbae Eggs (d) and Ascaridia galli Eggs (e).
|
Trichomonas gallinae (Protozoan) |
Eimeria labbeana (Coccidia) Oocysts |
Cryptosporidium meleagridis (Coccidia) Oocysts |
Ascaridia columbae (Nematoda) Eggs |
Ascaridia galli (Nematoda) Eggs |
|
|
Domestic Pigeons |
30 Infected/55 Birds |
15 Infested/55 Birds |
1 Infested/55 Birds |
9 Infested/55 Birds |
2 Infested/55 Birds |
|
Columba livia domestica |
54.54% a |
27.27% a |
1.81% a |
16.36% a |
3.63% a |
|
Rock Pigeons |
37 Infected/55 Birds |
13 Infested/55 Birds |
2 Infested/55 Birds |
12 Infested/55 Birds |
3 Infested/55 Birds |
|
Columba livia |
67.27% b |
23.63% b |
3.63% b |
21.81% b |
5.45% b |
|
Laughing Dove |
8 Infected/55 Birds |
17 Infested/55 Birds |
0 Infested/55 Birds |
10 Infested/55 Birds |
0 Infested/55 Birds |
|
Spilopelia senegalensis |
14.54% c |
30.90% a |
0.00% c |
18.18% a |
0.00% c |
|
Eurasian Collared Dove |
13 Infected/55 Birds |
14 Infected/55 Birds |
1 Infected/55 Birds |
8 Infected/55 Birds |
1 Infected/55 Birds |
|
Streptopelia decaocto |
23.63% d |
25.45% a |
1.81% a |
14.54% a |
1.81% a |
Table 3 Occurrence and prevalence of Trichomonas gallinae, oocystes and eggs of helminthes in different groups were shown. Different lower cases (a–d) show the significant difference between rows of every column. Values of (P≤0.05) were considered significant

Figure 7 Identified protozoan, oocysts and egg of helminths: the highest and lowest prevalence of each oocysts and egg of helminths were shown.
After the doing of three different types of qualitative tests on feces samples; namely direct smear, flotation and sedimentation techniques, the identified protozoan oocysts (Figure 6b&c) and helminthes eggs (Figure 6d&e) are listed in Table 4. There is significant difference between prevalence of identified oocystes and eggs of helminthes in different groups (Table 3). The highest prevalence of Eimeria labbeana (Coccidia) Oocysts is for Laughing Doves and the lowest prevalence of Eimeria labbeana Oocysts is for Rock pigeons, the highest prevalence of Cryptosporidium meleagridis (Coccidia) Oocysts is for Rock pigeons and the lowest prevalence of Cryptosporidium meleagridis Oocysts is for Laughing Doves, the highest prevalence of Ascaridia columbae (Nematoda) Eggs is for Rock pigeons and the lowest prevalence of Ascaridia columbae Eggs is for Eurasian collared Doves, the highest prevalence of Ascaridia galli (Nematoda) Eggs is for Rock pigeons and the lowest prevalence of Raillietina tetragona is for Laughing Doves (Figure 7).
|
Echinostoma revolutum (Trematode) |
Ascaridia columbae (Nematoda) |
Ascaridia galli (Nematoda) |
Raillietina echinobothrida (Cestode) |
Raillietina tetragona (Cestode) |
|
|
Domestic Pigeons |
9 Infested/55 Birds |
14 Infested/55 Birds |
2 Infested/55 Birds |
6 Infested/55 Birds |
5 Infested/55 Birds |
|
Columba livia domestica |
16.36% a |
25.45% a |
3.63% a |
10.90% a |
9.09% a |
|
Rock Pigeons |
11 Infested/55 Birds |
12 Infested/55 Birds |
4 Infested/55 Birds |
10 Infested/55 Birds |
7 Infested/55 Birds |
|
Columba livia |
20.00% a |
21.81% a |
7.27% b |
18.18% b |
12.72% a |
|
Laughing Dove |
3 Infested/55 Birds |
13 Infested/55 Birds |
0 Infested/55 Birds |
2 Infested/55 Birds |
3 Infested/55 Birds |
|
Spilopelia senegalensis |
5.45% b |
23.63% a |
0.00% c |
3.63% c |
5.45% b |
|
Eurasian Collared Dove |
5 Infected/55 Birds |
10 Infected/55 Birds |
1 Infected/55 Birds |
4 Infected/55 Birds |
0 Infected/55 Birds |
|
Streptopelia decaocto |
9.09% b |
18.18% b |
1.81% d |
7.27% c |
0.00% c |
Table 4 Occurrence and prevalence of endoparasites in different groups were shown. Different lower cases (a–d) show the significant difference between rows of every column. Values of (P≤0.05) were considered significant
After the evisceration process and examination of every group, some helminthes were removed from gastro-intestinal tracts of examined birds (Figure 8). Table 4 show the occurrence and prevalence of endoparasites in gastro-intestinal tracts of examined birds, the highest prevalence of Echinostoma revolutum is for Rock pigeons and the lowest prevalence of Echinostoma revolutum is for Laughing Doves, the highest prevalence of Ascaridia columbae is for Domestic pigeons and the lowest prevalence of Ascaridia columbae is for Eurasian collared Doves, the highest prevalence of Ascaridia galli is for Rock pigeons and the lowest prevalence of Ascaridia galli is for Laughing Doves, the highest prevalence of Raillietina echinobothrida is for Rock pigeons and the lowest prevalence of Raillietina echinobothrida is for Laughing Doves, the highest prevalence of Raillietina tetragona is for Rock pigeons and the lowest prevalence of Raillietina tetragona is for Eurasian collared Doves (Figure 9).
To the best of authors knowledge and current time this is the first study on the parasites in native resident species of Columbidae family in southwest of Iran, also it is one of the rare reports that compare the prevalence of different ectoparasites, endoparasites, protozoan, oocysts and eggs of parasites in different species of Columbidae family (Domestic pigeons, Rock pigeons, Laughing doves and Eurasian collared doves) simultaneously and at the same time in Iran and also in all over the world. In this study two different species of blood parasites identified in different examined groups: Haemoproteous columbae and Leukocytozoon marchouxi. In our study the highest prevalence of Haemoproteous columbae observed in Rock pigeons (27.27%), that is higher than its prevalence in last study of authors21 and it was lower than its prevalence in Mashhad, Iran.22 The prevalence rate for Hemoproteous spp. ranged from 20% to 32% in Queensland,23 Colombia,24 Bulgaria25 and the United States.26 The prevalence of blood parasite in pigeons and birds in Japan27, Costa Rica28 and Alaska29 was lower than 10%. Throughout the world prevalence of Haemoproteous columbae in feral pigeons in different geographical area varies from 14 to 100%.30 Yunus & Arsalan31 reported 74% of pigeons collected from a local zoo were infected with blood parasites. More-over, 105 Columba livia in Galapagos Island were investigated and 89% were infected with Haemoproteous spp.32
Leukocytozoon marchouxi is other blood parasites that identified in our study. Also the highest prevalence of Leukocytozoon marchouxi observed in Rock pigeons (5.45%), that is higher than its prevalence in Mashhad, Iran,22 also it is higher than its prevalence in hilly districts of Bangladesh.33 In our study this higher prevalence observed in Rock pigeons; maybe occur due to absence of anti-parasitic treatment in rock pigeons in their wild life. Different ectoparasites removed and identified in this study. Menopon gallinae, Pseudolynchia canariensis, Columbicola columbae and Lipeurus caponis identified that the highest prevalence of ectoparasites was for Columbicola columbae; observed in Rock pigeons (56.36%), that was more than its prevalence in Mashhad, Iran.22 Also the highest prevalence of Menopon gallinae, Pseudolynchia canariensis and Lipeurus caponis in our study was for Rock pigeon (21.81%, 36.36% and 16.36% respectively), that was more than their prevalence in Mashhad, Iran too.22 Other researchers found different prevalence of ectoparasites in their studies that were more or less than our results, for example: Dranzoa et al.34 found 94.1% prevalence of Columbicola columbae in Uganda. Menacanthus stramineus and Menopon gallinae was also found in the work of Dranzoa et al.,34 Msoffe et al.,35 Musa et al.,36 Adang et al.,37 Sari et al.,3 Marques et al.,2 Foronda et al.38 report different prevalence of ectoparasites in pigeons in different locations and times. Trichomonas gallinae identified in many birds of each groups, the high prevalence of this protozoan observed in Domestic pigeons and Rock pigeons (54.54% and 67.27% respectively): results of this study is in agreement with results of Al-Barwari and Saeed in Iraq:39 same as their study, the infection rate of Trichomonas gallinae is the highest among all the identified parasites in this study. Same as other scientists39 we think that since this flagellate protozoan is of cosmopolitan distribution and generally looked upon as a normal digestive tract inhabitant, then, as also mentioned by Levine40 and Soulsby,11 the vast majority of the older pigeons may be carriers of this organism. This means that they easily can introduce it to their young squads while feeding them the regurgitated holocrine substance or crop-milk. Trichomonas gallinae infections in rock pigeons were presented by other, for example, 11.0% from Chile,41 26.5% from Brazil42 and 32.0% from Italy.43
Eimeria labbeana Oocysts, Cryptosporidium meleagridis Oocysts, Ascaridia columbae Eggs and Ascaridia galli Eggs identified in feces samples: the highest prevalence of each of them in examined groups was 30.90%, 3.63%, 21.81% and 5.45% respectively. Prevalence of Eimeria labbeana in pigeons reported by Krontwald Junghanns et al.44 as 71.9%, in other study prevalence of Eimeria labbeana in pigeons reported by Mahdii and Al-Rubaie as 35% in Iraq.45 Results of our study is in against with result of above studies, we think that it is because of differences of climate and weather in Shahrekord that is very cold and dry in climate. The highest prevalence of Cryptosporidium meleagridis was 3.63% in our study, but in same study the prevalence of Cryptosporidium spp. reported 40% totally46 that may be the other spp. in this study made the prevalence of Cryptosporidium spp. different from our study. But in our study we could identify Cryptosporidium meleagridis only. Different amounts for prevalence of Eimeria labbeana Oocysts and Cryptosporidium meleagridis Oocysts reported in different studies such as: Nestro et al.,47 Radfar et al.,48 Fayer et al.,49 Ramires et al.,50 Mirzaei et al.,51 and Bomfim et al.52
In current study highest prevalence of gastro-intestinal helminthes: Echinostoma revolutum (Trematode), Ascaridia columbae (Nematoda), Ascaridia galli (Nematoda), Raillietina echinobothrida (Cestode) and Raillietina tetragona (Cestode) were identified 20%, 25.45%, 7.27%, 18.18% and 12.72% respectively. That the prevalence of each Ascaridida spp. eggs in feces samples was in agreement with prevalence of Ascaridida spp. helminthes in every groups. Results of our study are in agreement of results of Al-Barwari & Saeed,39 Marques et al.2 But our results are in against of results of Parsani & Momin,53 Begum & Shaikh54 who found 88.88% and 86% nematode infection, respectively. Moreover, higher prevalence of Ascaridia galli infection showed the similarity with the observation of Rabbi et al.55 There were many same studies on prevalence of gastro-intestinal helminthes in pigeons such as: Adang et al.,56 Ali et al.57 and Borghare et al.58
This study shows the prevalence of different ecto, endo, blood and protozoan parasites of 4 different native resident species of Columbidae family in southwest of Iran and also in all over the world simultaneously and at the same time. Results of this study help to every practitioners and researcher of this area to be conscious about occurrence and prevalence of parasites in pigeons in this area. Study on prevalence of parasites in pigeons in other locations in Iran and world and also in other species of pigeons and other family of birds that are native resident species in those areas can be considered for next studies.
Birds that examined in this study were the part of birds that prepared for thesis of Dr. Amir Dehghani-Samani. The Authors are grateful to the Deputy of Research, University of Shahrekord for financial support of this project.
Authors certify that there are no any financial and personal relationships with other people or organizations that could influence or bias their work and authors declare that there are no conflicts of interest.

©2020 Dehghani-Samani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.