Journal of
eISSN: 2377-4312


Research Article Volume 7 Issue 6
1Department of Veterinary Physiology & Biochemistry, Faculty of Animal Husbandry & Veterinary Sciences, Sindh Agriculture University Tandojam, Pakistan
2Department of Animal Nutrition, Faculty of Animal Husbandry & Veterinary Sciences, Sindh Agriculture University Tandojam, Pakistan
3Faculty of Veterinary and Animal Sciences, Lasbela University of Agriculture, Water and Marine Sciences, Pakistan
4College of Veterinary medicine, Nanjing Agriculture University, Nanjing, Jiangsu, 210095, China
Correspondence: Rong Rui, College of Veterinary medicine, Nanjing Agriculture University, China, Tel 0086-2584395595
Received: February 12, 2017 | Published: November 23, 2018
Citation: Abbasi B, Malhi M, Siyal FA, et al. Influence of dietary selenium yeast on fermentation pattern mucosal growth and glutathione peroxidase (gsh-px) activity in colon of goat J Dairy Vet Anim Res. 2018;7(6):253-259. DOI: 10.15406/jdvar.2018.07.00222
The present study was investigated the impact of dietary selenium yeast (SY) on the fermentation pattern and mucosal growth in the colon of goats. Ten cross-breed goats at 4 month of age and 10.5 kg BW were allotted in to two groups (n=5). The control group was fed basal diet, and treatment group receive basal diet with SY at the dose of 0.3 mg/kg feed. The result compare to control group revealed that blood Se concentration, and glutathione peroxidase (GSH-Px) activity were noted higher. Meanwhile the molar concentrations of propionate were noted higher in the colon of SY group which was manifested to increase in total SCFA concentrations and decrease the pH of colonic digesta compared to control. The weight of colon and colonic mucosa were improved significantly in SY goats compare to control. Moreover, histological findings of colonic wall showed that thickness of mucosa tunic and number of goblet cells per mm2markedly increased in treatment group compare to control. Additionally, GSH-Px activity in colonic epithelium significantly increased in the treatment group. Based on the findings it could be concluded that the dietary SY supplementation in diet of goats has significant impact to improved fermentation pattern, induced mucosal growth and increased GSH-Px activity in the colon of goats.
Keywords: selenium yeast, GSH-Px, colon, mucosal growth, goat
Selenium (Se) is a trace element essential for maintenance of normal health of animals and human. Selenium is required for biosynthesis of Selenoproteins such as glutathione peroxidase, which makes an important part of anti-oxidation system in animal body.1,2 The antioxidant properties of Selenoproteins help to prevent cellular damage from free radicals such as hydrogen peroxide, which are natural by-products of oxygen metabolism that may contribute to the damage of the cells.3,4 There are two main sources of Se i.e. inorganic and organic. Plants utilize inorganic Se in the form of selenite or selenite from soils, which is converted to, and stored in the organic form as Selenoproteins.5‒8 The Se status of soils in most parts of the world, including Pakistan is low, yielding Se deficient plants, leading to deficiency of Se in animal’s diet and eventually lead to lower Se concentration in animal products consumed by humans.9‒11 It is of immense significance that the animals must be supplemented with appropriate quantity of Se in ration. Sodium selenite and selenium yeast are vital resource of inorganic and synthetic organic sources of Se, regularly use in animal’s diet as supplements.12 The utilization and bioavailability of Se in animal body varies with the form of Se supplemented in animal’s diet. Previous studies showed that the absorption and availability of organic Se is higher in various tissues compared to those fed inorganic Sesupplementation.13,14 Moreover, the utilization and bioavailability of Se varies between ruminants and non-ruminants. The absorption of selenium in either form is significantly lower in ruminants than in non-ruminants. Mahan et al.,15 reported that absorption of supplemented organic Se was only 34% in sheep compared with 85% in swine. The low absorption of selenium in ruminants is due to its reduction to elemental (insoluble) form by ruminal microorganisms. Moreover, the inorganic Se sources (such as selenized yeast and Se-enriched probiotics) are more effective than inorganic Se sources (sodium selenite) in increasing tissue and blood Se concentrations as well as increasing GSH-Px activity in the blood.16 Selenium (Se) is an important antioxidant because itplay an important role in antioxidant enzyme regulation such as glutathione peroxidase17 and Se is essential for the efficient and effective operation of many aspects of the immune system in both animals and humans.18 Therefore the beneficial effects of organic selenium predominate over the inorganic selenium in ruminants.19 The intestinal mucosa contains a highly specialized tissue which differs in many aspects from other parts of the body.20 Colon is an important part of digestive tract which provides site for digestion as well as absorption of nutrients in the ruminants.21 The rate of transportation of nutrients from intestinal lumen into the peripheral parts of body depends upon normal intestinal health and mucosal surface area.22,23 The mucosal surface area depends upon the size and number of villi, which in turn depend upon the availability of essential nutrients including trace elements such as selenium. Dietary Se supplementation exhibited positively effects on intestinal health of animals. Previous study suggested that the Se has protective effects against intestinal infection in chickens.24 Dietary selenium supplementation has also been shown to increase digestibility of nutrients25 and improved growth performance,26 which indirectly suggest an increase transfer of nutrients through intestinal wall and indicate positive effects of selenium on intestinal growth in ruminants. Se deficiency is a serious problem for livestock production particularly in soil deficient part of the world. Previous research on ruminant proved that the significant impact of Se on growth and other aspects but literature is lacking information regarding the role of Se on colonic mucosa of goat. Therefore, the current study was carried out to investigate the impact of dietary selenium yeast (SY) on the fermentation pattern, mucosal growth and glutathione peroxidase (GPx) activity in the colon of goats.
All procedures used in this study were in accordance with international stander and guidelines. The experimental protocol was approved by Institutional Ethical Committee of the department of Animal Physiology and Biochemistry, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University Tandojam, Pakistan.
Animals, diet and experimental design
Ten healthy male cross breed goats, mean age 4 months and average initial body weight 10.5 kg, were purchased from small scale goat farm and brought at Livestock Experiment Station (LES), Department of Livestock Management, and Sindh Agriculture University Tandojam. Before commencement of the study, the goats were allowed for three weeks of adaptation period. During adaptation period the goats were ear tagged for identification, drenched against endo-parasite and vaccinated against some common infectious diseases. After adaptation the animals were randomly divided into two dietary treatment groups with five animals in each i.e. control (C) and selenium yeast (SY). Goats were brought from a location where the animal feed without Se supplementation. After allotting into groups, the treatment period followed for 8 weeks using a completely randomized design. According to the specific treatment, all animals were fed same basal diet (BD) consisting of guar hay and concentrate. The concentrate (150 g) was provided into two equal portions at 08:00 and 17:00 h daily and the hay and water was fed at ad libitum. The chemical analysis of basal diet is given in Table 1. The goats in control group were fed with basal diet whereas treatment group were given identical BD supplemented with additional 0.3 mg Se/kg feed respectively. The goats were housed in individual pens with bedding (1.65 m × 1.25 m).The treatment diets were prepared fresh daily, there is no dietary refusal and feed was completely consumed by the animals. At the end of 8thweek of experimental period, all goats were slaughter at Ruminant Research Unit, Sindh Agriculture University Tandojam. Immediately after slaughtering the abdominal cavity was opened and the colon was isolated from rest of the gastrointestinal tract, weight and size of the colon were recorded. After rinsing with PBS colonic tissues were collected for glutathione peroxidase (GSH-Px) activity and histomorphometry. In addition aliquot of 20-30 ml of colonic fluid was collected by straining the colonic digesta through two layers of cheesecloth. The pH was immediately measured by using pH meter. After addition of 5% HgCl2 (1mL/20mL of colonic fluid), the samples were stored at−20 °C for future analysis of short chain fatty acids (SCFA) Robert et al.27
Chemical composition |
Concentrate |
Hay |
Moisture |
12.98 |
10.85 |
Salt (NaCl) % |
0.38 |
0.23 |
Crude protein, % DM |
16.66 |
10.12 |
Crude fat, % DM |
10.75 |
6.7 |
Crude fiber, % DM |
9.23 |
19.82 |
Crude ash, % DM |
8.15 |
11.06 |
Gross Energy K/Cal |
4053.09 |
3446.35 |
Table 1 Chemical analysis of concentrates and hay fed to goats during experiment
The concentrate was composed of ground corn, soybean meal, cottonseed bran; wheat bran, fish meal, limestone, salt and vitamin premix (vitamin A, D and E).
Analytical procedures
Dry matter content of feed and samples were determined after drying at 105°C for 24 h in a forced air-drying oven AOAC 1990.28 Nitrogen contents of feed samples were tested with the Kjeldahl method AOAC 1990.28 Crude fat, crude fiber, ash and gross energy were measured according to AOAC 1990.28 The selenium content in the feed components, blood and tissues was analyzed fluorimetrically according to the method of Rodriguez et al.29 Serum GSH-Px activity was measured using glutathione peroxides enzymekit reagents (Nanjing Jiancheng Bioengineering Institute).
Histological examination
The histological study of colonic tissue was carried out according to the method as describe by Moolchand et al.30 Briefly the 1cm2tissue of colon was fixed in 4% par formalin solution in 0.1 M PBS for 24 hours. After rinsing with water, samples were stored in 0.1 moll-1 phosphate buffer (pH 7.2). Tissue was dehydrated in graded series of ethanol alcohol (30%, 50%, 70%, and 90%), cleared with xylem, saturated and embedded in paraffin. Tissue blocks were cut at 5-7 µm thickness and mounted onto glass slides. The sections were further cleared in xylem and rehydrated in descending grades of alcohol, washed in water and stained. After staining with the hematoxylin and eosin (H/E) stain, the sections were dehydrated in ascending grades of alcohol, cleared with xylem and finally, cover slipped with mounting media (Canada balsam). Five sections were determined for each goat. The morph metric procedure was carried out with a computerized image analysis program (DigiPro 4.0, Labomed, USA) on Hematoxylin and Eosin stained tissue sections under labomed digital microscope.
Statistical analysis
Data was analyzed by one-way ANOVA and significant level was compared by Tukeys method using SPSS-12 statistical software package. Data were presented as Mean ± SEM and differences were considered significant at P<0.05.
Serum Se concentration
The results showed the serum selenium (Se) concentration, after 8 weeks of dietary treatment, were significantly (P < 0.05) increased in goats received selenium yeast (SY) compare with control (Figure 1).
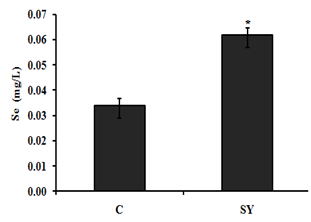
Figure 1 Effect of dietary selenium yeast supplementation on serum selenium concentration of goat. Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Data are presented, mean±SE significant difference at P<0.05.
Serum glutathione peroxides (GSH-Px) activity
In present study the glutathione peroxidase (GSH-Px) activity were assessed in goat serum, (Figure 2). After administration of dietary selenium yeast (SY), the results were compared to control group, it showed that glutathione peroxidase (GSH-Px) activity were significantly (P < 0.05) higher in treatment group.
Determination of short chain fatty acid (scfa) and pH in the colon of goat
In present study we quantified the lavel of molar concentrations of short chain fatty acids (SCFA) and pH to evaluate the effects of selenium yeast in the colon of goats, (Table 2). The result showed that the molar concentration of acetate and butyrate were not significantly (P>0.05) different, between SY and C groups, however the concentration of propionateincreased significantly (P<0.05) in SY goats compared to C group. The higher concentration of colonic propionate was manifested by the increase (P<0.05) in total SCFA concentration and significantly (P < 0.05) decrease the pH of colonic digesta of SY group compared with control.
Items |
Groups |
P-value |
|
C |
SY |
||
Acetate (mmol) |
52.34 ± 0.98 |
53.44 ± 0.89 |
0.427 |
Propionate (mmol) |
20.69 ± 0.43 |
26.01 ± 1.04 |
0.001 |
Butyrate (mmol) |
8.50 ± 0.42 |
8.30 ± 0.39 |
0.732 |
TSCFA (mmol) |
81.54 ± 1.14 |
87.76 ± 1.84 |
0.021 |
Ac: Pr Ratio |
2.53 ± 0.21 |
2.05 ± 0.18 |
0.632 |
pH |
6.27 ± 0.12 |
5.81 ± 0.11 |
0.025 |
Table 2 Effect of dietary selenium yeast supplementation on the molar concentrations of short chain fatty acids (SCFA) and the pH of the colon of goats
Ac: Pr = Acetate: Propionate Ratio Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Values (mean ± SE) differ at P < 0.05.
Wight of goat colon
The colon weight calculated in grams (g), percentage of final body weight (%FBW) and percentage of empty body weight (%EBW). The % FBW of colon were unchanged, however, the% EBW, were significantly (P>0.05) improved in SY group compared to C group (Table 3).
Items |
Groups |
|
P-Value |
C |
SY |
||
Colon (g) |
126.75 ± 0.85 |
135.50± 0.65 |
0.038 |
Colon (% FBW) |
0.90 ± 0.03 |
0.91± 0.03 |
0.734 |
Colon (% EBW) |
1.06 ± 0.00a |
1.11± 0.00b |
0.046 |
Table 3 Effect of dietary selenium yeast supplementation on weight of colon of goats
FBW, Final body weight; EBW, Empty body weight. Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Values (mean ± SE) differ at P < 0.05.
Size of goat colon
The size of colon calculated in centimeters per kilogram of final body weight (cm/kg. FBW) and centimeters per kilogram of empty body weight (cm/kg. EBW) (Table 4). The result showed that the size of colon cm/kg. FBW and cm/kg. EBW, whereon changed (P >0.05) between SY and C groups.
Items |
Groups |
|
P-Value |
C |
SY |
||
Colon, cm |
201.62 ± 5.85 |
217.53 ± 5.53 |
0.432 |
Colon, cm/kg.FBW |
14.70 ± 1.09 |
13.33 ± 0.34 |
0.814 |
Colon, cm/kg.EBW |
17.06 ± 0.88 |
16.54 ± 0.62 |
0.759 |
Table 4 Effect of dietary selenium yeast supplementation on size of colon of goats
FBW, Final body weight; EBW, Empty body weight. Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Values (mean ± SE) differ at P < 0.05.
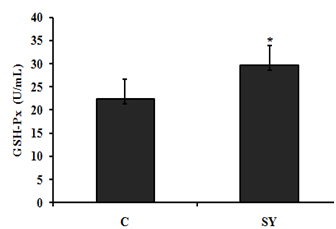
Figure 2Effect of dietary selenium yeast supplementation on serum glutathione peroxidase (GSH-Px) activity of goat. Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Data are presented, mean±SE significant difference at P<0.05.
Glutathione peroxides (GSH-Px) activity
Dietary selenium yeast significantly (P<0.05) improves the glutathione peroxidase (GSH-Px) activity in colonic epithelium of treatment group compare to control (Figure 3).
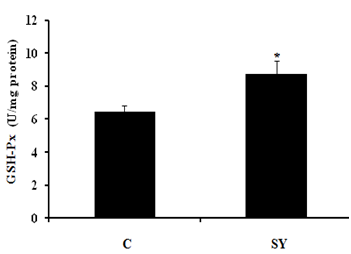
Figure 3Effect of dietary Se supplementation on glutathione peroxidase (GSH-Px) activity in colonic mucosa of goat. Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Data are presented, mean±SE significant difference at P<0.05.
Weigh of colonic mucosa of goat
The weight of colonic were significantly (P<0.05) improved in treatment group fed diet supplemented with SY compare to control group (Figure 4).
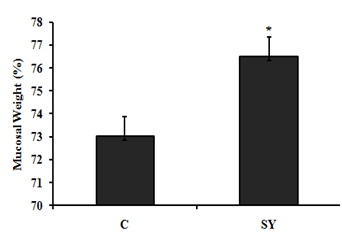
Figure 4Effect of dietary Se supplementation on mucosal weight (%) of colon of goat. Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Data are presented, mean±SE significant difference at P<0.05.
Histological examination
Our finding illustrated that SY supplementation (Figure 5) and (Table 5), the total wall thickness, thickness of muscle tunics and crypt depth were not influenced (P >0.05) by the dietary treatment, however, thickness of mucosa tunic and number of goblet cells per mm2 were improved (P<0.05) in colon mucosa of SY fed goats compare to control group.
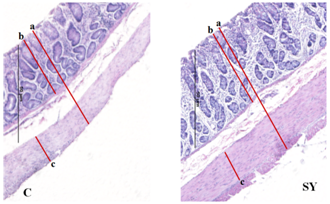
Figure 5Histomicrographs showing total wall thickness (a) thickness of mucosa tunic (b), and thickness of muscle tunic (c) in colon of goat. Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Data are presented, mean±SE significant difference at P<0.05.
Items |
Groups |
|
P-value |
C |
SY |
||
Total wall (µm) |
1047 ± 101.09 |
1354 ± 184.23 |
0.427 |
Muscle tunic (µm) |
20.69 ± 0.43 |
26.01 ± 1.04 |
0.001 |
Mucosa tunic (µm) |
8.50 ± 0.42 |
8.30 ± 0.39 |
0.732 |
Crypt depth (µm) |
81.54 ± 1.14 |
87.76 ± 1.84 |
0.021 |
Goblet cells (No./0.2 mm2) |
2.53 |
2.05 |
0.047 |
Table 5 Effect of dietary selenium supplementation on the histo-morphometry of colonic mucosa of goats
Goats were fed basal diet without Selenium supplementation (C, n=5) and Basal diet + selenium yeast (SY, n=5) for 8 weeks. Values (mean ± SE) differ at P < 0.05.
On the basis of the serum selenium (Se) concentration (mg/dl), the goats were categorized as Se deficient (0.006–0.03), Se marginal (0.031–0.06) and Se adequate (0.08–0.5). In the present study, the serum Se concentration of goats in control group was 0.034 mg/dl, which was close to the lower limit of marginal serum Se level and in SY group was 0.062 mg/dl, which was close to the lower limit of adequate serum Se level. The serum Se concentrations of goats in the present study reflect the dietary inclusion of Se fed to experimental animals. In control group, goats received basal diet containing 0.035 mg/kg Se. In SY group; the goats received basal diet supplemented with selenium yeast at the dose of 0.3mg/kg. In our study, the control group received Se deficient diet; the results are in agreement with.31 Who reported that the dietary SY were significantly improved serum Se concentration compared to those fed Se deficient diet. In addition, the previously published studies proven that the forage cultivation in Se deficient soil produced Se deficient forages and offer these forage to sheep which were found significantly lower blood Se level.10‒19 The increase serum Se concentration was associated with increase -serum glutathione peroxides (GSH-Px) activity in SY goats compared to control. Our findings are consistent with previous studies, reported that GSH-Px activity up regulated with dietary addition of organic selenium in goat.26‒32 Large intestine is divided into three parts i.e. colon, caecum and rectum. In addition to absorption of water and electrolytes, large intestine also provides the site for microbial fermentation especially in colon and caecum. Previous studies have shown that dietary Se supplementation improved fermentation by increasing the concentrations of acetate, propionate, and butyrate and eventually increase total SCFA in caecum and colon of rats.33 In current study, the molar concentration of propionate and total short chain fatty acid (SCFA) increased and the pH was decreased in the colonic digesta of goats supplemented with selenium yeast (SY) compared to the of control. To our knowledge, we have first reported the effects of SY on colonic fermentation in goats. However, dietary SY supplementation has been shown to increase propionate and total SCFA concentrations and decrease pH in colonic fluid of cattle fed SY supplemented diet.34,35 The increased SCFA concentrations suggest that SY improved microbial fermentation rate in the colon. The improved fermentation rate suggested that enhance the physiological efficiency of colon which revealed to increase mucosal mass.36 In present study, the improved fermentation was found to be associated with increase in colonic weight. Literature is lacking information regarding the effect of SY on colonic weight in goat; however, previous studies have shown that jejunal mass significantly increased in steers fed organic Se in the form of high-Se wheat.37 In current study, dietary SY supplementation did not alter the length of colon, however, macroscopic observations revealed comparatively thicker colonic wall in SY treated goats compare to control. This observation led us to hypothesize that increased colonic mass in SY fed goats might be due to increase the thickness of mucosal wall. Moreover, the intestinal wall is mainly composed of mucosal layer attached to the muscular layer; to increase intestinal length would require linearly increase both layers. Our findings suggested that the effects of SY supplementation would have been confined to one of these two layers that allowed colon to grow in thickness rather than in length. To confirm the hypothesis, we gently isolated mucosal layer from underlying musculature from middle of the colon and determined the mucosal weight. The results showed that relative mucosal weight were noted higher in SY treated goats compared to control. Previously publish studies not focus on the effects of dietary SY supplementation on mucosal weight of colon; however, Soto-Navarro et al.37 reported that the organic selenium in the form of high-Se wheat improve the weight of jejunal mucosa of steers. Histologically, the wall of colon, from the interior to the exterior, comprises four layers such as mucosa, sub mucosa, muscularis and serosa. The mucosal layer of colon consists of epithelium, lamina propria and muscular is mucosae. The epithelium forms a single layer of columnar epithelial cells and arranged into crypts full of goblet cells. The increased colonic mucosal weight in SY fed goats led us to speculate that dietary Se would have produced positive effects on histo-morphology of colonic mucosa. The results revealed that the thickness of mucosa tunic significantly increased in the colon of SY fed goats than that of control. Moreover, the increased mucosal thickness was associated with higher number of goblet cells per mm2, which suggest that SY supplementation induced hyper plastic effect on the colonic mucosa. The colonic mucosa is undergoing continuous renewal that occurs approximately every 3-5 days. This process is mediated by the stem cells located in the base of the crypts of colon, which undergo proliferation and differentiation to create different cell types. The new cells migrate towards the base or opening of the crypts, depending on the cell type. For example, secretary Paneth cells migrate down to the base of the crypts while mucus secreting goblet cells enter endocrine cells and absorptive epithelial cells migrate upwards along the surface epithelium. The amount of DNA in the tissue represents hyperplasia38 and the Se treatment increased DNA content in jejunal mucosa of steers and ewes, independent of its chemical nature and source. These data suggest that dietary Se induces hyperplasia which was further evidenced by higher number of proliferating cells in intestinal crypts of Se fed steers and sheep.37 Colonic epithelium is the most important barrier due to its numbers of reason importantly it provides the passage for SCFA absorption and also act as a demarcation between the external and internal environments. The excess oxidants in colonic epithelium are removed by most complex and active antioxidation defense mechanism.40 One of the antioxidant enzyme is a selenoprotein called glutathione peroxides (GSH-Px), found in gastrointestinal tract including colonic epithelium, which protects the tissue from damage by a powerful oxidant, hydrogen peroxide (H2O2) by converting it into water.41 In present study, we observed that the GSH-Px activity was increased up to 25.57% in treatment group compared to control. In addition to proliferative effects, the SY improved mucosal morphology by enhancing the protective mechanism through increased GSH-Px activity. Previously GSH-Px activity was not measured in colon of ruminants, in response to SY supplementation, however, the SY treatment showed variable response to GSH-Px activity in the intestinal mucosa of goats and rats.42,43 Our results contradicted with the findings of Chung et al.43 who did not observe any difference in the GSH-Px activity of intestinal mucosa of goats fed SY supplemented diet compared with control. In addition to great difference in the environments of small intestine and colon, the other possible reasons in this discrepancy may be the variation in dosage and duration of the experiment. The results of present study showed that the SY supplementation in diet exerted hyper plastic effect on colonic mucosa which may be due to its proliferative or protective effects. In summary, the present data demonstrated that the dietary SY supplementation in diet of goats improved fermentation pattern, induced mucosal growth and increased GSH-Px activity in the colon of goats.
The results of present study showed that the SY supplementation in diet exerted hyper plastic effect on colonic mucosa which may be due to its proliferative or protective effects. Dietary addition of selenium yeast (SY) could improve fermentation pattern, glutathione peroxidase GSH-Px activity, and histo-morphology by increasing mucosal thickness and number of goblet cells in the colon of goat.
There is no financial sponsor to this work. The authors are thankful to the supporting and technical staff of Livestock Experiment Station (LES), Department of Livestock Management, and Department of Animal Physiology and Biochemistry, Sindh Agriculture University Tandojam, Pakistan.
The author declares that there is no conflicts of interest.

©2018 Abbasi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.