Journal of
eISSN: 2377-4312


Mini Review Volume 3 Issue 3
1Department of Internal Medicine and Infectious Diseases, Mansoura University, Egypt
2Department of Clinical Pharmacy, Jazan University, Saudi Arabia
Correspondence: Mohamed Zakaria Sayed-Ahmed, Department of Internal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura 35516, Egypt, Tel 00966 594 886878, Fax 00966 17 321 6837
Received: February 26, 2016 | Published: May 12, 2016
Citation: Sayed-Ahmed M. Incidence history of west Nile virus in Africa and middle east, with an emphasis on Egypt: a review. J Dairy Vet Anim Res. 2016;3(3):101-104. DOI: 10.15406/jdvar.2016.03.00080
Importance: Since its initial isolation in Uganda in 1937 and its introduction in Egypt in 1950 through the present, West Nile virus (WNV) have had a significant global public health impact during the last decades due to their resurgence and dynamic epidemiologic features in human and animals, and its considered one of the largest arboviral neuroinvasive disease recorded in the world.
Objective: To review the incidence of West Nile virus in both animals and human, with an emphasis on Egypt, to follow the preventive measures to reduce the public health impact of WNV in areas with the potential for transmission.
Background: WNV has a history of irregular but recurrent epizootics in countries of Africa, Middle East and Central, Eastern and parts of southern Europe. WNV is an enveloped virus of the genus Flavivirus, is naturally maintained in an enzootic cycle between birds and mosquitoes, with occasional epizootic spillover causing sporadic cases and outbreaks of human and equine. It has recently gained considerable attention as re-emerging infections in a global scale. Environmental factors (heavy rains followed by floods, irrigation, high temperature, or mass breeding of mosquitoes) that enhance population densities of vector mosquitoes could increase the incidence of WNV infection. The disease gets its name from the West Nile District of Uganda in Africa where it was first recognized in humans in 1937. The first cases of WNV in horses were recognized in France and Egypt in the 1960’s. Since WNV is a biosafety level 3 agent, techniques that involve cell culture are restricted to laboratories with this level of biosafety, such as reference laboratories. Nevertheless, outbreaks of WNV remain unpredictable. Further coordinated studies are needed for a better understanding of the epidemiology of the virus.
Conclusion: In order to fully understand the impact of WNV and/or other flavivirus infections in Egypt, epidemiology and ecological features of these agents need to established. West Nile virus has and will remain a formidable clinical and public health problem for years to come.
Keywords:west Nile virus, incidence, bird migration, neurological disease, Egypt
WNV, west nile virus; PFU, plaque forming units; SLEV, saint louis encephalitis virus; JEV, japanese encephalitis virus; CDC, centers for disease control and prevention
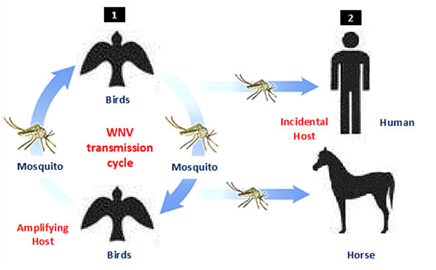
Since the West Nile virus (WNV) strain (B956) was isolated in 1937 from a clinical case was suffered from fever in the West Nile district of Uganda, WNV start to appear again in 1950s causing different infrequent outbreaks in different continent of the world including Egypt, Israel, France and India.1‒6 In the past 78years since its discovery, the disease has continued to increase in vast areas of the world to consider the main cause of viral encephalitis in the world. WNV is an emerging zoonotic arbovirus widely distributed throughout the world with both considerable impacts on public and animal health.7 The virus maintained in enzootic cycle involving mosquito’s vector and certain birds as reservoir hosts (Figure 1). There are many vertebrate hosts other than birds such as reptiles, amphibians, and mammals are susceptible to WNV infection.8 Although, humans and horses (incidental hosts) are not able to develop sufficient viremia to infect mosquito, and do not participate in the WNV lifecycle, it may undergo severe signs or death from infection. The virus was replicates in a wide range of mosquitoes and transmitted exclusively among avian species.2 Culex mosquitoes are infected during sucking viremic blood (approximately 104-105PFU/mL) Numerous reports have suggested that WNV may persist in the organs of birds in such a way as to permit retransmission to vector mosquitoes after the period of initial viremia.9
WNV history in Africa and Middle East
In 1939-1940, a serosurvey was conducted in Uganda, Sudan, Congo, and Kenya to compare the neutralizing titers for WNV, SLEV (Saint Louis encephalitis virus) and JEV (Japanese encephalitis virus). This study revealed widespread seropositivity of WNV over 50%.10 In 1951 and 1955, the samples collected from patients in western Nigeria showing seropositivity for WNV.11 In 1954, seropositivity to WNV was demonstrated in animals, humans, monkeys, and birds in South Africa.12 WNV was isolated for first time during outbreak near Haifa in Israel from a febrile child in 1951.13 In 1952 and 1953, subsequent WNV outbreaks occurring mainly in human in Israel and the virus was isolated from human and chickens.14‒16 In 1980 and 2000, several WNV outbreaks attacked Israel with around 400 serologically confirmed cases and around 35deaths.17 Since that time, Israel has the experience to regulate and predict the annual summertime outbreaks, similar to those observed in the United States.18,19 WNV was reported in Turkey in the 1970s and 2000s,20‒23 followed by an epidemic reported in 2010-11, including Turkey and area of Mediterranean.24 In Iran, there many WNV cases were recorded in the 1970s, 2008s, and 2009s of patients suffered from fever, and some nervous sign, these cases were identified by RT-PCR and presence of specific IgG in their blood.25,26
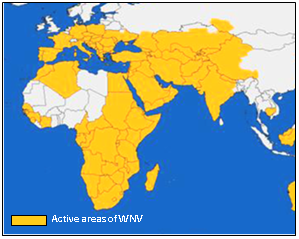
In 2008-2009, a study was conducted on horses in Iran to identify WNV antibodies, the results revealed the highest activity of virus in western and southern provinces with seroprevalences up to 88% in some areas.27 Although, no human cases have been reported, animal infections with WNV were recorded in Jordan and Lebanon.28‒31 In the late 20th and early 21st century, outbreaks of WNV continued to infect humans and/or horses in different localities in northern and sub-Saharan Africa (Figure 2) with active transmission in Morocco in 2010 and Tunisia in 2012 and ongoing sporadic transmissions in Algeria and Egypt.32‒41 In 2006, recent reports have indicated ongoing WNV transmission in Guinea,42 Ghana in 2009,43 and fatality cases in Gabon in 2009.44 In 2009, seroconversion of sentinel chickens was observed in Senegal.45 In 2010, the first case of lineage 1 WNV was recorded in South Africa causing death of a pregnant mare.46 In 2010-2011, human and mosquitoes positive for WNV lineage 2 were reported in eastern Africa (Djibouti).47
History of WNV in Egypt
WNV was first isolated in Egypt in 1950 in north of Cairo, where serosurveys was conducted of 251 individuals; mostly children showed that 22% of children and 61% of adults had antibodies to WNV.3 There are several large outbreaks were recognized in Egypt between 1951 and 1954, which led to a further understanding of the epidemiology of WNV.3,4 A study conducted in the Alexandria Fever Hospital in 1968 revealed that WNV infection was the etiology in 14.6% of the children admitted to the hospital with a febrile illness.6 In 1989, a seroprevalence study conducted in the Nile Delta showed only 3% prevalence of WNV in school children aged 8-14years.48 In 1999, a preliminary serological study demonstrated the WNV was widely distributed in Egypt (NAMRU-3, unpublished data). Around 75% of the human infections with WNV are mostly asymptomatic, and less than 1% of infections progress to severe disease varies from flu-like to severe neurological symptoms. WNV infection causing morbidity and mortality in birds. In Egypt, prior to 1998, the only evidence for virulence of WNV in naturally infected bird was the isolation of the virus from pigeon.49
Since its isolation in Africa in 1937, WNV has considered one of the most prevalent causes of arboviral neuro invasive disease in the world. Recent serious outbreaks of WNV in Africa and Middle East prove the risk of disease transmission with mosquitoes, the need to understand the epidemiology in endemic areas and the need for continues investigation and preventative measures. Few data exist regarding long-term sequelae from WNV infection, the risk of virus transmission associated with blood transfusion, potential therapeutic interferences, and vaccine possibilities. Physicians engaged in diagnosing and reporting WNV infection may greatly enhance knowledge of this emerging infectious disease.
None.
Author declares that there is no conflict of interest.

©2016 Sayed-Ahmed. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.