Journal of
eISSN: 2377-4312


Research Article Volume 1 Issue 2
1Department of Veterinary Sciences and Public Health, University of Milan, Europe
2Department of Drug Sciences, University of Pavia, Europe
Correspondence: Massimo Faustini, Department of Veterinary Sciences and Public Health, University of Milan, Via Celoria, 10, 20134 Milan, Europe, Tel 39 02 50317938
Received: September 05, 2014 | Published: October 31, 2014
Citation: Faustini M, Torre ML, Perteghella S, et al. Fatty acid composition, fat globule size and reactive oxygen species-scavenging activity of mare milk: a longitudinal study. J Dairy Vet Anim Res. 2014;1(2):37-44. DOI: 10.15406/jdvar.2014.01.00010
Mare milk is taken into account as a substitute for cow milk in case of allergy, especially in infant nutrition due to a composition similar to human milk. The aim of the work is to define some mare milk characteristics during lactation, from foaling to the 4th month of lactation. All the parameters considered to describe fat globule dimensions vary significantly during the lactation period. Fatty acid composition generally changes within the first days of lactation and after 42-56 days, with different behavior when considering medium or higher carbon chains. The ROS-scavenging activity expresses a positive trend and reaches the zenith at day 84. Finally there is a number of correlations between fat globules dimensional parameters and fatty acid composition and between long chain unsaturated fatty acids and ROS-scavenging activity, while dimensional parameters and ROS-scavenging activity are not statistically related. Results could be useful in veterinary and human field as milk substitutes.
Keywords: mare, milk, fatty acid profile, milk fat globule, ROS-scavenging activity
CM, cow milk; MM, mare milk; HM, human milk; ROS, reactive oxygen species; TAC, total antioxidant capacity; d4,3, volume-weighted diameter; d10, percentiles of d4,3 distribution; d50, percentiles of d4,3 distribution; d90, percentiles of d4,3 distribution; SSA, specific surface area; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; UFA, unsaturated fatty acids; CLA, conjugated linoleic acid; MFGM, milk fat globule membrane.
The nourishment of a newborn totally depends on the intake of mother milk. Milk not only provides the adequate nutrient and water supply, but also the transfer of immunity against pathogens from mother to the newborn.1 In humans, when mother’s milk is not available, babies are usually fed with cow’s milk (CM) implying the risk of developing allergies. Milk allergies account for an incidence of 2.5 % in young children, but often disappear within 3 years after birth; however, this time lapse shows an increasing trend.2 In these cases, milk replacers with characteristics close to breast milk are required. Even the results on equine milk tolerance are not still complete,3 Businco et al.4 evidenced that mare milk (MM) could be used as a good substitute to CM because it did not induce, in most cases, adverse reaction in children with severe IgE- and non IgE-mediated CM allergy. Furthermore, MM is considered highly digestible, rich in essential nutrients, with an optimal whey protein/casein ratio and, thanks to its flavor, it is attractive to children.3,5 In human species, equine milk consumption is diffused in Asia: this practice dates back to about 2000BC in Chinese records;6 the most part of mare’s milk is consumed by humans under the form of a lactic-alcoholic beverage called koumiss, very common in central Asia.7
In Western Europe, instead, MM is generally studied only to better understand its role in the foal growth.8 Many studies have been focused on the composition of MM sometimes making comparison with cow and human milk (HM). MM has a lower energy value than cow milk or HM, due to less fat content, but it has similar lactose percentage with respect to HM and higher than CM; MM and HM are lower in whole proteins and salt content than CM.9 Despite a plethora of results for CM and HM fat constituents, the composition of MM fat have been occasionally analyzed, mainly when different periods of lactation are considered.10,11 The literature regarding MM fat characteristics basically lacks a deep analysis of milk fat globule dimensional parameters: the importance in determining such values is not only linked to a commercial aspect, as reported for bovine dairy products,12 but also to physiological cues, since the catalytic capacity of pancreatic lipase is strictly connected to the fat globule dimension and the presence of a phospholipid membrane.13
Milk provides, besides immunoglobulins and other protection factors, such as antioxidants and reactive oxygen species (ROS) scavenging molecules. The total antioxidant capacity (TAC) of milk is the result of a complex interaction between partially known antioxidant components, such as catalase, superoxide dismutase, glutathione peroxidase, carotenoids and vitamins A, C, and E.14 The relation between milk TAC and newborn health status seems to be somewhat strong. Ledo et al.15 report that human milk protects, although partially, from the oxidative stress associated with prematurity in babies, and the milk formulas provide less antioxidant power than breast milk.14 Some studies are based on the evaluation of antioxidant potential of HM16 and CM,17 but little is known about the MM potential and the effect of period of lactation on it.
The composition of milk strongly changes during lactation, so that one can argue about three types of milk from the beginning to the end of lactation: colostrum, transition milk and mature milk. It seems that mare’s colostrum shows difference with milk just in the first two days of lactation. From day 2 to 5 after foaling, milk is considered as transitional, and after that period it is considered as mature milk.18 In order to evaluate its potential in infant nutrition, MM should be investigated for better understands how its characteristics and properties change during lactation.
In this study, milk collected from mares until the 4th month of lactation were considered and analyzed for the fatty acid composition, milk fat globule dimensional parameters and ROS‑ scavenging activity, aiming to better characterize MM under several biological aspects.
Preparation of mare milk samples
Milk samples were collected from 7 lactating mares belonging to four different breeds: Tiropesanterapidoitaliano (n=2); Sellaitaliano (n=3); Argentinian (n=1); Haflinger (n=1). All horses were in good health condition, had eutocic foaling and no oxytocin was administered before parturition or milking. All mares were maintained with a lactating mare diet. Diet was composed, for the whole follow-up period, of 60% hay and 40% concentrate. Concentrate composition is reported in detail in Table 1.
Component |
Percentage |
Rolled oats |
44.5 |
Wheat bran |
10 |
Soybean meal |
10 |
Molasses |
7 |
Dicalcium phosphate |
1 |
Limestone (ground) |
1.5 |
Trace mineralized salt |
0.5 |
Vitamin premix |
0.5 |
Nutrient content |
|
Crude protein |
14.5 |
Ca |
0.9 |
P |
0.6 |
Table 1 Mares concentrate composition in percentage
Milk samples were collected on the foaling day (1st day) and on the 3rd, 5th and 7th days of lactation. After the first week samples were collected once a week until day 28th of lactation. Starting from the 2nd month, samples were collected every 14days till 112th day post partum was reached. Milk samples were collected in 10mL test tubes and forwarded to the laboratory under controlled temperature (4°C). The determination of milk fat globule dimension and of the ROS-scavenging activity were performed within 4 hours, while for the other analysis samples were stored at -20°C until analysis.
Milk fat globule granulometry
Granulometric screening of milk fat globules was performed by a laser light scattering granulometer (Beckman Coulter LS230, equipped with a small volume cell, 120ml volume chamber, obscuration 5%) with refractive index set at 1.330 for water. Milk samples were directly put drop-wise into the measurement cell and ran in 5 replicates of 90 seconds each. Samples were evaluated for the mean volume-weighted diameter (d43, μm), specific surface area (SSA, cm2/mL of globules), the 10th, 50th, and 90th (μm) percentiles of d43 distribution (d10, d50, d90, respectively), and the span index, a dimensionless index of diameter dispersion, calculated as (d90‑d10)/d50*100.
Fat extraction and fatty acid analysis
The milk fat content of each sample was determined by the UV spectrophotometrical method proposed by Forcato et al.19 briefly, 30-60μl of milk are added of ethanol and stored for a time >1h at -20°C in order to precipitate interfering proteins and peptides. The supernatant is read at 208nm wavelength in a UV/VIS spectrophotometer.
Milk fat was extracted from the thawed samples by the method described by Bligh & Dyer20 modified by Manirakiza et al.21 Briefly, 1mL of each sample was transferred in a 15mL Falcon test tube and 3.75mL of chloroform: methanol 1:2 solution was added. After 10minutes of vortex processing, 1.25mL of chloroform was added, and the sample was vortex-stirred for 1min. Finally, 1.25mL of microfiltered, distilled water was added and the resulting suspension was vortexed again for 60'.
Samples were centrifuged at 2000rpm for 10minutes at 20°C in order to obtain the separation of three phases: the highest density phase, clear, composed of fat dissolved in chloroform; the intermediate density one, mainly composed of proteins, and the lowest density phase (aqueous medium).
The chloroform-containing phase was filtered with paper and collected in a previously weighted test tube containing anhydrous sodium sulfate, in order to eliminate possible water residues. One hundred and fifty µL of nonadecanoic acid (C:19) were added to each sample as an internal standard for gas chromatography. The solution was dried under mild nitrogen flow and the solid matter quantified.
Samples prepared as described above, were stored at -80°C until GC analysis was performed. For this purpose, the sample was dissolved in chloroform: methanol 2:1 solution with a ratio of 1mL for 1mg of extract.
Sample were derivatized as described by Moltó-Puigmartí et al.22 with some modifications: each sample was incubated with 0.5mL of sodium methoxide at 80°C for 10minutes, then cooled at 37°C, prior to a new incubation with 0.5mL of boron trifluoride at 80°C for 3minutes; finally, the sample was cooled at room temperature.
After the adding of 0.5mL of hexane, samples were shaken for 1minute, then 0. mL of NaOH saturated solution and anhydrous Na2SO4 were added to obtain 3 distinct phases. The supernatant was collected, dried under mild nitrogen flow and suspended in 1mL of hexane. 0.5µl of this solution were injected in a gas chromatograph Trace GC 2000 Series equipped with a Teknokroma column (100mT plus C max 150/prog 250°C, High Polar, Flow N2 0,5ml/min (0,25 mm x 0,20µm Film) and a pre-column of 5mT (ID = 0,25) deactivated). A programmed temperature run was used: the initial oven temperature was 70°C, isothermal for 2’30”. Then, temperature was increased at a rate of 3°Cmin−1 to 240°C. The total analysis time was 95min. Gas-chromatographic peaks were identified by comparing the retention times of sample with a 37 fatty acid methylester mix (FAME Mix 37, Supelco). For each sample at each day of lactation was evaluated the percentage of the following fatty acids: octanoic (C8), decenoic (C10), lauric (C12), myristic (C14), myristoleic (cis-9) (C14:1), pentadecanoic (C15), palmitic (C16), palmitoleic (cis-9) (16:1), heptadecenoic (cis-10) (C17:1), stearic (C18) , oleic (cis-9) (C18:1c), elaidic (trans-9) (C18:1t), linoleic (cis-9,12) (C 18:2c), γ-linolenic (cis 6,9,12) (C18:3n6), α-linolenic (cis-9,12,15) (C18:3n3), eicosanoic (C20), eicosaenoic (cis-11) (C20:1), eicosadienoic (cis-11,14) (C20:2), eicosatrienoic (cis-11,14,17) (C20:3n3) and 9-11 conjugated linoleic acid (CLA 9-11).
The total percentages of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA) and the unsaturated fatty acids (UFA) were calculated. The ratios between unsaturated and saturated fatty acids were calculated as MUFA/SFA, PUFA/SFA, and UFA/SFA.
ROS-scavenging activity
Milk samples were analyzed for the ROS-scavenging activity through the method described by Zarban et al.,16 with some modification; 100µl of each sample was added to 2mL of 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma-Aldrich) in methanol solution (100mM). After incubation at 37°C for 30minutes in dark conditions, 1mL of chloroform was added and the resulting solution was centrifuged at 3500rpm for 10minutes. The absorbance was evaluated at 515nm wavelength through a UV/VIS spectrophotometer (Uvikin 930, Kontron Instruments). Determinations were repeated in triplicate and averaged. A methanol solution of DPPH was used as control and the percentage of DPPH radical scavenging was calculated with the following equation:
Where, Ctr is the absorbance of the control, Sample stands for the absorbance of the sample and Blank is the absorbance of a blank solution (100µL sample + 2mL methanol + 1mL chloroform).
Data analysis
All data were resumed by mean±standard deviation. Results obtained from granulometric analysis, ROS-scavenging potential and fatty acid percentages were statistically analyzed through a one-way repeated measures analysis of variance (ANOVA) followed by the Tukey post hoc test for multiple comparisons, considering the day of lactation as fixed value.
Aiming to explore the relations between fat globule dimensional variables, ROS-scavenging power and fatty acid content, a product-moment correlation was applied, and all variables were submitted to a principal component (PCA) analysis; the PCA evaluation was performed on normalized data, after varimax rotation.
Repeated measures ANOVA was performed with the software SAS ver. 9.1 for Windows platform; correlation analysis and PCA were performed with the software Statistica ver. 7 for Windows platform.
Fat globule dimensions
The results of fat globules granulometric analysis are reported in Figure 1. The statistical analysis of the mean volume-weighted diameter of fat globules d4,3, Figure 1a shown significant differences (p=0.0001) as function of day in milk; in particular, it rapidly decreases during the first days of lactation and particularly between day 1 and day 5. After that period, the d43 decreases less steeply from day 58 to day 112 (not significant). The SSA (Figure 1b) changes significantly its values during time (p=0.0033); after an initial oscillatory phase, it increases from day 56 to the end of follow up. The mean values of d10, d50, d90 (Figure 1c) vary significantly during lactation (p<0.01 for all three variables): it must be stressed that the most significant changes in these variables occur during the first week of lactation. The d10 and the d90 increase and decrease, during the first week of lactation, with a “funnel shaped” pattern (Figure 1c). The d50 decreases significantly in the first week of lactation, and maintains somewhat constant levels during the follow up (Figure 1c). The steep variations in the diameters of the fat globules during the first period of lactation reflect on the span (Figure 1d) that decreases significantly in the first days after parturition, almost halving its values after 5days.
Fatty acid composition
The milk fat percentage, for all subjects during the whole follow-up period ranged between 0.07% and 5.25%, with mean values of 2.02±0.95%. An oscillating pattern until day 14 (maximum mean value 3.24±1.06% on day 7) is observed. From this day mean values significantly decreased constantly during time according to the following equation (pslope=0.0007; r2 =0.872):
The patterns of the 20 fatty acids during the 112 days of lactation are reported (mean values) in Table 1. All fatty acids changed significantly in percentage: their mean values during the follow up period, except for C8 and C16:1 cis. It must be noted that several long-chain fatty acids, such as C18:3n3, C18:3n6, and C20:3n3 raise their values starting from 42-56days of lactation, whereas fatty acids with shorter carbon chains (C14 and C14:1) tend to reduce their percentage along the lactation development. All the derived values (SFA, MUFA, PUFA, UFA), and the fatty acid calculated ratios (MUFA/SFA, PUFA/SFA, and UFA/SFA) significantly vary during time (Table 2). The sum of PUFA and UFA significantly raise their mean values, starting from the 56th day of lactation; a noteworthy increase (about 10%, p<0.01) in UFA and PUFA can be seen between the 42th and 56th day of lactation. The increase in UFA and PUFA is then counterbalanced by a dramatic decrease in SFA during the same period.
|
Day in milk |
|
|||||||||||||
Fatty acid |
1 |
3 |
5 |
7 |
14 |
21 |
28 |
42 |
56 |
70 |
84 |
98 |
112 |
SEM |
p |
(mean %) |
|||||||||||||||
C8 |
0.98 |
1.85 |
1.82 |
1.5 |
1.44 |
1.73 |
1.28 |
2.04 |
1.5 |
1.52 |
2.46 |
2.48 |
1.1 |
0.11 |
n.s. |
C10 |
4.93 |
8.15 |
7.45 |
7.06 |
6.82 |
7.29 |
6.61 |
7.13 |
4.93 |
4.69 |
6.01 |
6.95 |
3.98 |
0.24 |
0.007 |
C12 |
5.46 |
8.99 |
8.23 |
8.77 |
8.56 |
8.82 |
7.84 |
8.53 |
5.53 |
5.49 |
6.55 |
7.44 |
4.34 |
0.27 |
0.006 |
C14 |
6.8 |
9.23 |
9.54 |
10.02 |
9.49 |
9.29 |
8.25 |
8.43 |
5.92 |
6.16 |
6.23 |
7.22 |
5.44 |
0.23 |
<0.0001 |
C14:1 |
0.32 |
0.58 |
0.61 |
0.58 |
0.57 |
0.6 |
0.53 |
0.59 |
0.37 |
0.43 |
0.51 |
0.53 |
0.38 |
0.02 |
0.007 |
C15 |
0.36 |
0.33 |
0.39 |
0.35 |
0.3 |
0.33 |
0.32 |
0.25 |
0.25 |
0.34 |
0.37 |
0.36 |
0.33 |
0.01 |
0.04 |
C16 |
27.31 |
27.34 |
27.9 |
29.89 |
27.73 |
26.13 |
24.18 |
23.87 |
21.92 |
22.48 |
21.07 |
21.17 |
25.28 |
0.39 |
<0.0001 |
C16:1 |
5.87 |
6.3 |
6.46 |
6.49 |
7.11 |
7.19 |
6.32 |
6.93 |
5.88 |
5.92 |
5.91 |
5.42 |
7.57 |
0.15 |
n.s. |
C17:1 |
0.35 |
0.22 |
0.23 |
0.24 |
0.22 |
0.2 |
0.24 |
0.17 |
0.17 |
0.21 |
0.21 |
0.22 |
0.23 |
0.01 |
0.006 |
C18 |
3.16 |
2.33 |
2.71 |
2.43 |
1.94 |
1.66 |
1.7 |
1.44 |
1.47 |
1.57 |
1.26 |
1.21 |
2 |
0.08 |
<0.0001 |
C 18:1c |
24.58 |
19.07 |
19.95 |
17.21 |
18.58 |
19.05 |
18.88 |
18.85 |
19.92 |
20.86 |
20.21 |
18.76 |
24.25 |
0.51 |
0.05 |
C18:1t |
0.01 |
0.01 |
0.01 |
0.01 |
0 |
0 |
0.02 |
0.03 |
0.02 |
0.03 |
0.02 |
0.02 |
0.01 |
0.002 |
0.008 |
C 18:2c |
8.61 |
7.43 |
7.1 |
8.21 |
9.23 |
9.33 |
9.23 |
9.21 |
10.88 |
11.23 |
11.63 |
10.88 |
10.23 |
0.2 |
<0.0001 |
C18:3n6 |
0.46 |
0.13 |
0.03 |
0.06 |
0.08 |
0.08 |
0.09 |
0.04 |
0.08 |
0.12 |
0.08 |
0.08 |
0.18 |
0.02 |
<0.0001 |
C18:3n3 |
10.03 |
7.71 |
7.35 |
7.05 |
7.28 |
7.27 |
10.3 |
11.11 |
19.85 |
17.93 |
15.86 |
15.06 |
13.87 |
0.67 |
<0.0001 |
C20 |
0.11 |
0.02 |
0.03 |
0.03 |
0.02 |
0.02 |
0.03 |
0.03 |
0.02 |
0.03 |
0.03 |
0.02 |
0.05 |
0.004 |
0.005 |
C20:1 |
0 |
0.01 |
0 |
0 |
0.01 |
0.01 |
0.02 |
0.04 |
0.05 |
0.09 |
0.24 |
0.19 |
0.03 |
0.01 |
<0.0001 |
C20:2 |
0.19 |
0.07 |
0.07 |
0.15 |
0.16 |
0.16 |
0.2 |
0.18 |
0.18 |
0.23 |
0.24 |
0.21 |
0.19 |
0.01 |
<0.0001 |
C20:3n3 |
0.28 |
0.17 |
0.11 |
0.19 |
0.18 |
0.17 |
0.24 |
0.26 |
0.32 |
0.38 |
0.35 |
0.35 |
0.32 |
0.02 |
0.0002 |
CLA 9-11 |
0.05 |
0.04 |
0.04 |
0.03 |
0.05 |
0.09 |
0.07 |
0.07 |
0.04 |
0.04 |
0.02 |
0.06 |
0.02 |
0.004 |
0.0142 |
Table 2 Fatty acid profile during the follow up period. The p-values for ANOVA are reported
ROS-scavenging activity
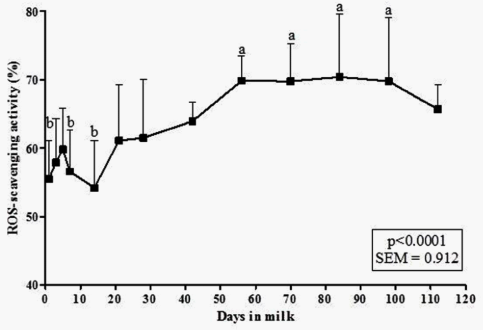
The 112day pattern of ROS-scavenging activity is reported in figure 2. The repeated measure ANOVA stressed out a significant difference during the sampling times (p<0.0001). The activity slightly increases from day 1 to day 5 and after it decreases till day 14 when reached the lowest average value (48.9%). The ROS-scavenging activity then raised its values reaching percentage of about 70% from the 56th day of lactation, maintaining a significant plateau with respect to the first 14days in milk until the 98th day in milk. Overall, the mean ROS-scavenging activity during the whole follow-up was 62.38±9.85%(n=91).
Relations between dimensional fat globule parameters, ROS-scavenging activity and fatty acid percentage
In order to explore possible relations between the dimensional parameters, the ROS-scavenging activity and the percentage of fatty acids in the mare milk samples, a bivariate correlation analysis was performed, followed by a principal component analysis. Table 3 shows the Pearson correlations for these variables.
|
Day in milk |
|
|||||||||||||
Index |
1 |
3 |
5 |
7 |
14 |
21 |
28 |
42 |
56 |
70 |
84 |
98 |
112 |
SEM |
p |
SFA (%) |
49.54 |
58.47 |
58.37 |
60.38 |
56.61 |
55.55 |
50.53 |
51.96 |
41.82 |
42.6 |
44.43 |
47.22 |
42.83 |
0 |
<0.0001 |
MUFA (%) |
30.89 |
26.17 |
27.1 |
24.34 |
26.38 |
27.02 |
25.84 |
26.51 |
26.29 |
27.35 |
26.92 |
24.94 |
32.26 |
0.58 |
n.s. |
PUFA (%) |
19.64 |
15.6 |
14.95 |
15.72 |
17 |
17.18 |
20.19 |
20.91 |
31.43 |
30.08 |
28.29 |
26.72 |
24.87 |
0.81 |
<0.0001 |
UFA (%) |
50.53 |
41.78 |
42.05 |
40.06 |
43.38 |
44.21 |
46.03 |
47.42 |
57.72 |
57.43 |
55.21 |
51.65 |
57.13 |
0.91 |
0.042 |
MUFA/SFA |
0.63 |
0.47 |
0.48 |
0.41 |
0.47 |
0.5 |
0.52 |
0.51 |
0.64 |
0.66 |
0.64 |
0.54 |
0.77 |
0.02 |
0.0006 |
PUFA/SFA |
0.4 |
0.27 |
0.26 |
0.27 |
0.3 |
0.32 |
0.4 |
0.42 |
0.77 |
0.74 |
0.66 |
0.59 |
0.6 |
0.03 |
<0.0001 |
UFA/SFA |
1.04 |
0.74 |
0.74 |
0.67 |
0.78 |
0.82 |
0.92 |
0.93 |
1.42 |
1.39 |
1.29 |
1.13 |
1.37 |
0.04 |
<0.0001 |
Table 3 acid classes and indices during the follow up period. ANOVA p-values are reported. SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; UFA, unsaturated fatty acids
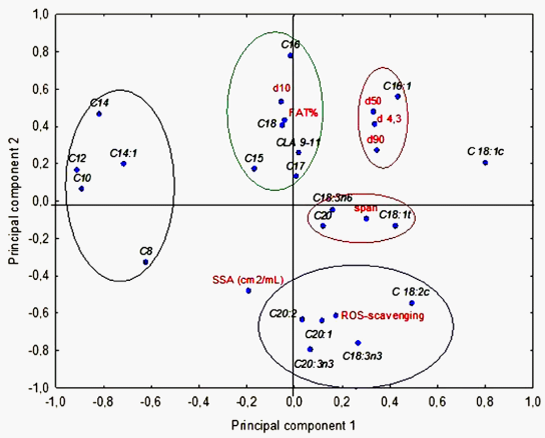
At a first glance, it appears that the dimensional parameters, as d4,3, d10, d50 and d90 are negatively correlated to the percentages of the shortest carbon chain fatty acids, and positively related to the longest chain ones, with some exceptions (e.g. C20:3n3); the same observations can be drawn for d4,3. The ROS-scavenging activity shows significant negative correlations with medium-chain saturated fatty acids (C14, C16, C18), and moderate positive correlations with several unsaturated fatty acids (C18:1t, C18:2c, C18:3n3, C20:1, C20:2 andC20:3n3). These complex relations can be advantageously explored by the PCA procedure: (Figure 2) reports the projection of the loadings for the variables on the space of the first two PCs; multivariate analysis put in evidence the close relation between fatty acid percentage and ROS-scavenging activity/dimensional parameters (Table 4). The first two components explain the 45.17% of the total variability. The fatty acids C18:2c, C18:3n3, C20:1, C20:2 and C20:3n3 form a cluster around the ROS-scavenging activity position in the PC space, whereas CLA 9-11, C15, C16, C17 and C18 are negatively related to the ROS-scavenging activity and positively linked to the fat percentage and the d10.
Variable |
C8 |
C10 |
C12 |
C14 |
C14:1 |
C15 |
C16 |
C16:1 |
C17:1 |
C18 |
C18:1t |
C18:1c |
C18:2c |
C18:3n6 |
C18:3n3 |
C20 |
C20:1 |
C20:2 |
C20:3n3 |
CLA9‑11 |
d 4,3 (μm) |
-0,33 |
-0,24* |
-0,25* |
-0,11 |
-0,35* |
0,19 |
0,23* |
0,05 |
0,45* |
0,46* |
0,02 |
0,44* |
-0,11 |
0,47* |
-0,19 |
0,17 |
-0,19 |
-0,08 |
-0,22* |
0,23* |
SSA (cm2/ml) |
0,15 |
0,04 |
0,03 |
-0,06 |
0,15 |
-0,12 |
-0,13 |
-0,02 |
-0,23* |
-0,24* |
0,02 |
-0,17 |
0,11 |
-0,06 |
0,15 |
0,12 |
0,14 |
0,20 |
0,24 |
-0,17 |
d10 (μm) |
-0,01 |
0,21 |
0,28* |
0,36* |
0,19 |
0,01 |
0,21 |
0,14 |
-0,06 |
-0,05 |
-0,04 |
-0,15 |
-0,17 |
-0,38* |
-0,25* |
-0,33* |
-0,15 |
-0,32* |
-0,31* |
0,08 |
d50 (μm) |
-0,31* |
-0,21 |
-0,20 |
-0,07 |
-0,30* |
0,18 |
0,24* |
0,10 |
0,38* |
0,39* |
0,05 |
0,40* |
-0,09 |
0,38* |
-0,23* |
0,14 |
-0,19 |
-0,14 |
-0,27 |
0,25* |
d90 (μm) |
-0,34* |
-0,30* |
-0,33* |
-0,20 |
-0,41* |
0,19 |
0,19 |
-0,01 |
0,49* |
0,51* |
0,02 |
0,48* |
-0,07 |
0,58* |
-0,13 |
0,24* |
-0,16 |
0,01 |
-0,15 |
0,19 |
Span |
-0,27* |
-0,35* |
-0,41* |
-0,35* |
-0,44* |
0,11 |
0,04 |
-0,11 |
0,41* |
0,40* |
0,06 |
0,44* |
0,03 |
0,56* |
0,06 |
0,28* |
-0,03 |
0,16 |
0,07 |
0,07 |
ROS-scavenging(%) |
0,17 |
-0,08 |
-0,17 |
-0,37* |
-0,09 |
-0,07 |
-0,59* |
-0,13 |
-0,19 |
-0,41* |
0,24* |
-0,01 |
0,41* |
-0,13 |
0,42* |
-0,10 |
0,45* |
0,38* |
0,39* |
-0,16 |
Table 4 Correlations between dimensional parameters of milk fat globules, ROS-scavenging milk activity and percentage of fatty acids in milk samples (n=7). Asterisks denote correlations with p<0.05
Differences in composition reflect the requirement of the newborn and may be connected to different breed besides day of lactation.18 In our study we did not analyze the influence of breed on the considered value because of the small number of animal of the same breed involved.
Fat globule dimensions
Lipids in milk are organized in dispersed globules surrounded by a complex membrane, called the milk fat globule membrane (MFGM). In the mammary epithelial cell the accumulation of lipids within the endoplasmic reticulum (ER) causes the growth of the lipid droplets. From ER they are first transported to apical cell regions and then secreted. It is known that MFGM have both technological and nutritional properties, i.e. it is the emulsifying agent for lipid in aqueous phase.23 The structure of human and mare membrane fat globules are similar, since they show three phospholipid layers, with an oligosaccharide structure on the outer side.9 About globules dimension, our results, especially regarding d4,3 and d90, are in partial agreement with those obtained by Doreau & Martuzzi.24 As a matter of fact, on day 1 fat globules are slightly over 3µm but thereafter they show a lower dimension, between 2 and 3µm, as reported also by Malacarne et al.9 HM fat globules have an average diameter of 4µm1 while for CM it is of 3-5µm. Dimension of fat globules are very important for lipid digestion by human pancreatic lipase (hPL) as discovered by Berton et al.13 In particular, the catalytic efficiency of hPL is 4.6-fold higher on small (1.8µm) than large (6.7µm) native milk fat globules, so MM should be more easily to digest than CM because of smaller fat globules dimension.
Fat content
Milk composition reflects the requirement of nutrients of the newborn. MM has a lower fat content than HM and CM,9 therefore the energy value is lower, besides the high lactose content.3 Even though Doreau25 reported a range of 1-2% in fat, in the present study, milk had an average fat content of 2.02%, significantly influenced by the day of lactation. From day 21 the total fat content followed a significant linear negative trend. This result is in contrast with that reported by Salimei & Fantuz3 who indicated only a negative trend without significant difference from day 20 to 150 of lactation.
Concerning the fatty acid composition, besides the single patterns, a general temporal trend can be defined: percentage of almost all fatty acids changed within the first three days of lactation, and after 42-56days. The latter changes are quite opposite when medium (C10-C15) or higher (>C15) carbon chains are considered: the first show a decreasing trend, while the latter percentage increased , in some cases in a dramatic fashion, such as in case of C18:3n3, C18:3n6 and C 20:1. CLA percentage follows an intermediate pattern with respect to medium/long chain fatty acids.
The differences observed in MM composition are consistent with the opinion that mare colostrum differs only in the first day from mature milk, although some results obtained in this study are in partial disagreement with those reported by Pikul &Wojtowski,26 who found that SFA and UFA in colostrum were about 40% and 60%, respectively, while we obtained approximately 50% and 50%; on the other side, the same percentage (about 0.05%) was found for the CLA. The same authors report higher percentages of C17:1, C20:1 and C20:2, whereas C14 shows lower level only in the first period of lactation. C18:3n3 percentage was lower with respect to that reported by Pikul & Wojtowski26 but higher than the mean value referred by Salamon et al.18 Finally, the percentages C12, C16:0 and C16:1 found in this study differed from the value as reported by Pikul & Wojtowski26 in terms of temporal significance.
A number of differences can be appreciated between MM, CM and HM composition. With respect to the values as reported by Salamon et al.18 and Jensen & Newborg27 for CM, shorter chain saturated fatty acids, as C8, C10 and C12, and longer chain unsaturated fatty acids, such as C16:1, C18:1c, C18:2c and C18:3n3 reported in the present study show higher percentages when compared to CM; on the other side, the percentages of C14, C14:1, C15, C17:1, C18, C18:3n6 and C18:1c resulted lower. C16 yielded discordant results between the reported studies; our data are closer to those published by Jensen & Newborg27 but lower than those referred by Salamon et al.6
By comparing the results of the present work with those reported by Uniacke-Lowe & Fox6 and by Darragh & Lönnerdal28 on HM, some differences emerge; saturated shorter chain fatty acids (C8, C10 and C12) are more abundant in MM than in HM, whereas heavier saturated fatty acids (C18 and C20) are appreciably lower in MM. A parallel behavior cannot be evidenced in unsaturated fatty acids: three medium/high molecular weight unsaturated fatty acids (C16:1, C18:3n3 and C20:3n3) are higher in MM than in HM, whereas C17:1, C18:1c, C18:1t, C18:3n6, C20:1 resulted lower in MM.
All these discrepancies between the considered studies could be due to scheduling differences in collecting samples.
ROS-scavenging activity
After foaling, the newborn is subjected to oxidative stress stimuli, because of the transition from intrauterine environment to the external one. Breast milk could protect against oxygen free radical damage because of its antioxidant and ROS-scavenging activity.29,16 To better understand if MM could be administered as an alternative to HM, we evaluate the ROS-scavenging activity of MM samples. Our results suggest that the activity shows a positive trend during lactation, and the maximum value is reached at day 84. These results are similar to that obtained for CM antioxidant activity by Kankofer & Lipko-Przybylska30 but in contrast with those reported for HM by Zarban et al.16 using DPPH method, according to which the colostrum expressed the higher ROS-scavenging activity. Therefore, if the use of MM as HM replacer aims to limit the effects of ROS species in the newborn, the administration of a mature MM (i.e. between 56 and 100days of lactation) should be recommended.
Relations between dimensional fat globule parameters, ROS-scavenging activity and fatty acid percentage
In the present study the mean ROS-scavenging activity of MM is higher than in HM. In particular, the mean value obtained is of 62.38±9.85% (n=91) while for humans varies from 50.4±19.7% in colostrum to 44.6±18.5% in mature milk.16 Furthermore, bovine milk was found to have a mean ROS-scavenging activity of 55.6±4.87%(n=33). From the results obtained in our study, the long chain UFA C18:2c, C18:3n3, C20:1, C20:2 and C20:3n3 seem to be related to the ROS-scavenging activity of MM. In particular, C18:3n3 percentage is almost tenfold higher in MM than in HM, and could have an active role in conferring the ROS-scavenging activity. However, the ROS-scavenging activity was evaluated on the raw milk so that fatty acids may contribute only to a part of the total activity.
As expected, a number of correlations between fat globules dimensional parameters and fatty acid percentages were noted. These correlations could be due to a different composition along the range of size distribution. As a matter of fact, the same behavior was observed also for HM31 and CM.32
PCA basically confirms the results of correlation analysis, with a cluster of long chain UFA loadings close to the ROS-scavenging activity in the space of the first two PC. The SSA seems to be quite positively related to the ROS-scavenging activity although the r coefficient (0.17) was not statistically significant.
In this study some peculiar characteristics of mare milk as a function of lactation period were analyzed. The results could be useful either in veterinary practice for the formulation of equine milk artificial replacers or in human nutrition, considering mare milk as a substitute for human milk, even if a full standardization of the product is difficult due to the natural variability of the considered parameters. Mare milk is endowed of characteristics similar to human milk (e.g. fat globule dimensions) thanks to which it could be considered as a valid human milk replacer also in cow milk intolerance. Furthermore, in advanced lactation (between 50 and 100days), mare milk could be administered to support the intrinsic ROS-scavenging activity. To better characterize mare milk, further studies could aim to evaluate the parameter differences between breeds.
Massimo Faustini and Daniele designed the experiment and wrote part of the paper; Maria Luisa Torre, Theodora Chlapanidas and Sara Perteghella conducted the granulometic and the ROS scavenging analysis and interpreted the results; Carla Colombani and Eleonora Munari performed and interpreted the fatty acid analysis; Luca Maria Chiesa supervised the fatty acid results.
None.
Author declares that there is no conflict of interest.

©2014 Faustini, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.