Journal of
eISSN: 2377-4312


Research Article Volume 10 Issue 1
1Pathology Department, Faculty of Veterinary Medicine, Mansoura University, Egypt
2Virology Department, Mansoura University, Egypt
Correspondence: Walaa F Awadin, Pathology Department, Faculty of Veterinary Medicine, Mansoura University, Egypt
Received: February 11, 2021 | Published: March 30, 2021
Citation: Awadin WF, El-Tholoth M. Diagnostic studies on some infectious diseases affecting skin in bovine and buffaloes calves at EL-Dakahlyia province, Egypt. J Dairy Vet Anim Res. 2021;10(1):11-17 DOI: 10.15406/jdvar.2021.10.00305
Background: Infectious cutaneous diseases are major threat of bovine and buffaloes calves in Egypt and cause huge economic impact as a result of hide damage, decreased growth rate and mortality rate.
Material & methods: A retrospective study was conducted between October 2016 and January 2020 to identify different infectious diseases affecting skin in 5000 examined bovine calves from ten local dairy farms located at El-Dakahlia province. Clinical signs, morbidity and mortality rates were reported. Gross examination of lesions was applied. Skin biopsies were collected from lesion and surrounding areas and part of these samples fixed in 10% buffered formalin for routine hematoxylin and eosin (H&E) staining. Periodic acid Schiff stain (PAS) and immunohistochemistry (IHC) were also applied for diagnosis. The other part of skin samples was kept at-80°C for viral isolation by real time- polymerase chain reaction (RT-PCR).
Results: Four infectious diseases were detected in 2220 examined bovine calves out from 5000 (44.4%) including warts (150 cattle calves), FMD (1500 cattle calves and 500 buffaloes calves), LSD (45 cattle calves and 5 buffaloes calves) and ring worm (20 cattle calves). Special staining using PAS was sufficient to diagnose ring worm. Histopathology and IHC diagnosed few cases infected with LSD that showing inclusion bodies in 10 out of 50 calves (0.2%). No inclusion bodies were seen in any infected specimens with FMD or papillomas. Viral nucleic acids of FMD, papillomatosis and LSD virus were detected in infected skin samples.
Conclusion: cattle calves showed higher susceptibility to these infectious skin diseases than buffalo calves. FMD caused higher morbidities than in other diseases. Mortalities were reported with FMD and LSD infection. Histopathology was not sufficient to diagnose viral infection. IHC cannot diagnose LSDV in all infected cases. PCR should be used for accurate detection LSD, FMD and papilloma viral nucleic acids in skin samples.
Keywords: LSD, foot and mouth disease, ring worm, calves, skin, diagnosis
Skin diseases have significant economic losses due to degradation of hides and skin, reduction of wool quality, meat and milk yield, losses due to culling and occasional mortalities, as well as, cost of treatment and prevention of the diseases.1,2 In addition, some skin diseases such as ringworm and sarcoptic mites have potential zoonosis.3,4 The most common cattle skin diseases reported in Ethiopia were dermatophilosis, lumpy skin disease (LSD), dermatophytosis, pediculosis, acariasis, photosensitization and warts.5,6 In a study conducted by Fantaye and Melake7 revealed that 146 out of 662 cattle examined were positive for different skin problems such as ring worm, lice infestation, tick infestation, wart, LSD, manage and dermatophilosis. LSD outbreak was reported in Egypt after importation of Ethiopian animal in 2006. LSD epidemics were recorded in Ethiopia during 2005.8 Prevention of animal transport from infected country is very important to avoid treatment costs and high economic losses resulting from hide damage, loss of milk due to mastitis and loss of animal products due to deaths, abortion, fever and myiasis.9 In a cattle farm at Ismailia province northeastern Egypt, clinical cases of foot and mouth disease (FMD) were first recognized on January 22, 2006.8 Initial testing with antigen enzyme linked immunosorbent assay and real time- polymerase chain reaction (RT-PCR) assays suggested that multiple FMD virus (FMDV) serotypes may have been involved in the outbreak, although only type A was later confirmed.8 The lack of disease reporting pre-importation or at the quarantine stations obstacles the authorities in controlling the disease. Because imported animals may acquire infection at any point up until their arrival, they must be vaccinated and tested for the absence of FMDV nonstructural proteins.8 In spite of feasible control measures, they are timely implemented because the encountered skin diseases continuously varied causing adverse effects on cattle production, tanning industry and public health. This study aimed to identify major infectious viral skin diseases of cattle and buffaloes in local dairy farms at El-Dakahlia province, Egypt.
Animals & tissue specimens
A total of 5000 bovine calves (cattle & buffaloes) of both sexes were examined in 10 local dairy farms at EL-Dakahlyia Province, Egypt during the period October and January 2017-2020 for presence of cutaneous lesions. All suspected animals were clinically examined. The animal examination was concentrated on physical statutes, temperature, and superficial lymph node according to Radostits et al.10 After external examination, skin biopsies were taken from the lesion and surrounding tissue. Additional samples were collected from grossly affected animals in necropsied recently dead calves. One Part of skin, as well as, heart specimens was fixed in 10% neutralized formaldehyde. The other part of skin specimens was kept at-80°C for viral isolation by RT-PCR.
Study area
All laboratory procedures were carried out at Pathology and Virology Departments, Faculty of Veterinary Medicine, Mansoura University during the period October and January 2017-2020.
Histopathological and immunohistochemistry (IHC) examination
Formalin fixed tissue samples were routinely processed in ascending grades of alcohol, xylol, embedded in paraffin wax, sectioned at 4–5μm, and stained with alcohol, xylol, embedded in paraffin wax, sectioned at 4–5μm, picked up on negatively charged slides and stained with hematoxylin and eosin (HE) or periodic acid Schiff stain (PAS) for histopathologic examination according to Bancroft and Gamble.11 Other formalin fixed paraffin embedded tissue samples were cut and picked up on positively charged slides for immunohistochemistry (IHC) examination. Paraffin sections were deparaffinized in xylene, rehydrated in ascending grades of ethyl alcohol then washed with PBS. H2O2 was placed on tissue sections for blocking of endogenase peroxidase. Antigen retrieval was carried on by placing tissue sections in 1:10 target retrieval solution (pH 9; Dako) in microwave adjusted at 200W for 20 min according to Awadin et al.12 After rinsing the slides in PBS, 1: 500 diluted monoclonal antibody F80G5 that was generated using E. coli expressed capripoxvirus ORF 057, a viral core protein, was used for the detection of LSDV antigen monoclonal antibody was applied to cover the sections and the slides were incubated at 37°C for 1 h. EnVision anti-mouse HRP (Dako) and universal secondary antibody (Dako) were applied then slides were incubated at 37°C for 30–45min. The employed chromogen substrates was 3, 3-diaminobenzidine (ImmPACTTM DAB) with HRP-immunoperoxidase method for 5–10min. Sections were counterstained with Mayer’s hematoxylin and then dehydrated, cleared and mounted. The prepared sections were examined under light microscope.
Nucleic acids extraction
RNA extraction from FMDV suspected samples was done using QIAamp Viral RNA Mini Kit (Cat. No. 52906) according to the manufacturer’s instructions. Normal non-infected samples were included as a negative control sample. DNA extraction of bovine papillomatosis virus (BPV-1) was performed as reported previously by Viljoen et al.13
Oligonucleotide primers
The used oligonucleotide primers were designed for specific real time amplification of FMDV, BPV-1 and LSDV.14–16 Oligonucleotide primers were synthesized by Sigma Chemical Company, USA. The primers were received in lyophilized form and resuspended in Tris/EDTA buffer to reach a final concentration of 100pmol/μL.
qRT-PCR amplification for FMDV
TenμL reaction volume used for identification of FMDv. The reaction composed of 1.5μL of extracted RNA, forward and reverse primers (10μM), 6μL of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), 0.2μL of reverse transcriptase, AMV (each1μL contain 200U ) (Promega) and nuclease free water to a total volume of 10μL. The cycling conditions were as follows: a cycle at 50ºC for 20 min then incubation for 10 min at 95ºC and then 45 cycles of 95ºC for 10s and 60ºC for 30.
qPCR amplification for BPV-1 and LSDV
The reaction is consisted of 1.5μL of extracted RNA, forward and reverse primers (10μM), 6μL of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), and nuclease free water to a total volume of 10μL. The cycling conditions were as follows: Incubation at 95ºC for 2 min then 45 cycles of 95ºC for 10s and 60ºC for 30s.
Four infectious skin diseases were detected in 2220 examined bovine calves out from 5000 (44.4%) including: LSD in 45 cattle calves (0.9%) and 5 buffaloes calves (0.1%), FMD in 1500 cattle calves (30%) and 500 buffalo's calves (10%), warts in 150 cattle calves (3%) and ring worm in 20 cattle calves (0.4%) as shown in (Graph 1).
Clinical signs of LSD were fever, decreased body weight, increased salivation in 5% of calves lachrymation, conjunctivitis in 2% of calves, single or multiple circular skin nodules of variable sizes (from 7 millimeters to 2–4 cm in diameter) were seen in all infected calves (Figure 1a-c). Swollen superficial lymph nodes were also noticed in all infected calves Mortalities reached (30%). In necropsied recently dead calves, subcutaneous areas of congestion and fibrosis (Figure 1d). Lungs of dead calves showed chronic interstitial pneumonia (Figure 1e) with presence of circular to oval grey nodules (Figure 1f), hydrothorax and fibrinous pleurisy. Swollen mediastinal, prescapular and prefemoral lymph nodes were detected in dead calves. Spleen of dead calves was slightly enlarged and congested. Histopathological analysis of nodular skin lesions showed hyperkeratosis, epidermal acanthosis, hydropic to severe ballooning degeneration, eosinophilic intracytoplasmic inclusion bodies (Figure 2a) only in 10 (0.2%) bovine calves (8 cattle and 2 buffaloes), dermal edema, hyperemia, mononuclear cell infiltration and vasculitis (Figure 2b) appear in all infected animals with variable severity. Lungs of dead calves showed non-suppurative interstitial pneumonia characterized by thickening of interstitial tissue with mononuclear cells infiltration (Figure 2c, 2d). Well demarcated necrotizing areas are seen in lung tissue (Figure 2e, 2f). Lymph nodes of dead calves showed lymphoid hyperplasia, marked congestion (Figure 2g) and perivascular oedema (Figure 2h). The spleen of dead calves displayed lymphoid hyperplasia, congestion and hemorrhage. IHC using monoclonal antibodies against Neethling virus revealed strong positive brown staining in epidermal cells dermal macrophages and fibroblasts (Figure 2i) and in lymphocytes in superficial lymph nodes (Figure 2j) in 10 calves showing intracytoplasmic inclusion bodies (0.2%). Meanwhile, negative staining to weak positive brown reaction appeared in dermal macrophages and fibroblasts in other cases.
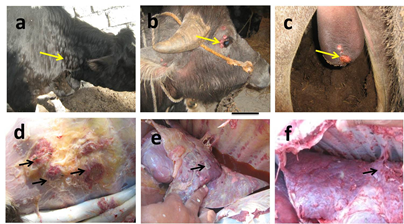
Figure 1 Gross picture of LSD infected calves showing multiple circular skin nodules of variable sizes in neck in cattle calves (yellow arrows) (a), in face around eyes eyelids (yellow arrows) (b) and on scrotum (yellow arrows) (c) in buffalo calves. Gross picture showing subcutaneous areas of congestion and fibrosis (black arrows) (d), chronic interstitial pneumonia with presence of circular to oval grey nodules (black arrow) (e), fibrinous pleurisy (black arrow) (f) in necropsied recently dead calves.
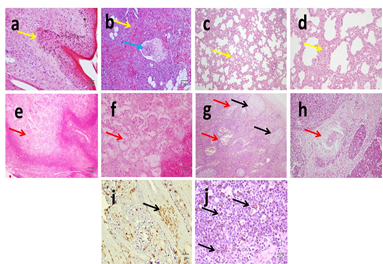
Figure 2 Histopathological analysis of H&E stained skin slides from LSD showing hyperkeratosis, epidermal acanthosis, hydropic to severe ballooning degeneration, eosinophilic intracytoplasmic inclusion bodies (yellow arrows) (a), dermal mononuclear cell infiltration (yellow arrow) and vasculitis (blue arrow) (b). Histopathological analysis of H&E stained lung slides from LSD shows non-suppurative interstitial pneumonia characterized by thickening of interstitial tissue with mononuclear cells infiltration (yellow arrows) (c&d). Well demarcated necrotizing areas are seen in lung tissue (red arrows) (e&f). Histopathological analysis of H&E stained lymph node slides from LSD shows lymphoid hyperplasia (black arrows), marked congestion (red arrows) (g) and perivascular oedema (yellow arrow) (h). IHC using monoclonal antibodies against Neethling virus revealed positive brown staining in epidermal cells, dermal elements (i) and in lymphocytes in superficial lymph nodes (j) X:40 (a,b,c,e,g); X:100 (d,f,h,i); X:400 (j).
Clinical signs of FMD were fever, ropy salivation (Figure 3a) with slobbering, smacking of lips and lameness in all infected calves. Skin vesicles and ulcers were detected on nostrils (Figure 3b), muzzle, gum (Figure 3c), tongue (Figure 3d), feet in 100% of calves and on teats in 50% of calves. The mortality rate was very low (5%) except in suckling animals reaching (100%) as a result of myocardial damage. In those cases, inappetence to anorexia, dullness incoordination, recumbency, tachycardia and tachypnea were recognized prior to death. Yellowish (Figure 3e) to grayish (Figure 3f) streaking bands were observed on myocardium of dead calves giving the characteristic tiger heart appearance. Histopathological examination of skin of muzzle revealed focal destruction of epithelial and subepithelial structures with the presence of inflammatory cells, which reveals lesions of necrotic and ulcerative dermatitis. No inclusion bodies can be detected in any infected calves. Myocardial hyalinization and coagulative necrosis was observed in dead calves with interstitial edema and mononuclear cells infiltration (Figure 3g).
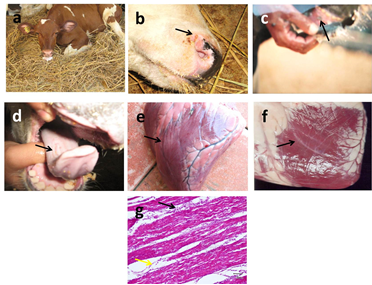
Figure 3 Gross pictures of FMD infected calves salivation (a), vesicles and ulcers on nostrils (black arrow) (b), muzzle, gum (black arrow) (c), tongue (black arrow) (d), yellowish (e) to grayish (f) streaking bands (black arrows) on myocardium. Histopathological examination of H&E stained myocardium shows interstitial edema (black arrows) and mononuclear cells infiltration (yellow arrows) (g). X:100.
Warts appeared on calves as solitary and multiple solid nodules and cauliflowers covered (Figure 4a) with dried thick horny layer without fever. No mortalities were reported. They frequently appeared on head & neck (30%), back (15%), limbs (50%) and might be also generalized (0.5%). Histopatholgical examination of warts all infected calves revealed hyperkeratosis, acanthosis, elongated rete-pegs, intact basement membrane with fibrous stroma (Figure 4b). No inclusion bodies can be detected in any infected animal.

Figure 4 Gross pictures of generalized skin papillomatosis on calves (a). Histopatholgical examination of warts revealed hyperkeratosis (black arrow) acanthosis (red arrow), elongated rete-pegs (blue arrow) with fibrous stroma (asterisk) (b). X:40.
Ring worm appeared in all infected calves as circular areas of alopecia with varying size on the face, neck, and back of the animal (Figure 5a, 5b) Histopatholgical examination of skin biopsies affected with revealed hyperkeratosis, acanthosis (Figure 5c) with presence of small round basophilic bodies (ectothrix arthrospores) inside hair follicles around damaged hair shaft in H&E stained slides (Figure 5d) and eosinophilic septated hyphae in PAS stained slides (Figure 5e).
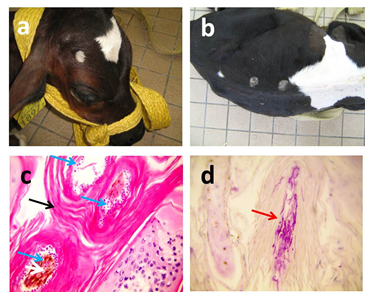
Figure 5 Gross pictures of ring worm in calves appeared as circular areas of alopecia in face (a) and back (b) of the animal. Histopatholgical examination of H&E stained skin biopsies shows hyperkeratosis (black arrow) with presence of small round basophilic bodies (ectothrix arthrospores) inside hair follicles around damaged hair shaft (blue arrows) (c). Eosinophilic septated hyphae (red arrow) appear in PAS stained slides (d). X:400.
Ct values of extracted nucleic acids showed positive amplification signals for LSDV (Figure 6a), FMDV (Figure 6b) and BPV-1 (Figure 6c) were conducted in clinically collected samples from diseased animals. Non template control and negative controls did not show any amplification results.
LSD, FMD, papillomatosis and dermatophytosis were diagnosed in ten local dairy farms at El-Dakahlia province with higher susceptibility of cattle calves than buffalo calves.
LSD is an economically important infectious disease of cattle.10 LSDV as well as sheeppox and goatpox viruses belong to the genus Capripoxvirus within the subfamily Chordopoxvirinae of the family Poxviridae.17 Collectively, these viruses cause the most serious poxvirus diseases in production animals. LSD is characterized by rapid eruption of multiple circumscribed skin nodules, generalized lymphadenopathy and fever.10 LSD can be histologically differentiated from urticaria, insect and tick bites or insect stings by the absence of eosinophils and the presence of a deep vasculitis, cutaneous lymphosarcoma, streptothrichosis and tuberculosis by the presence of eosinophilic inclusion bodies in keratinocytes and germinal cells of affected hair follicles and sebaceous glands in early lesions.18 Eosinophilic intracytoplasmic inclusion bodies were detected only in 10 bovine calves, so molecular diagnosis seems to be essential for assuring infection. In our findings, gross and microscopic pathology besides IHC staining of LSD were similar to those previously described by Awadin et al.12 In addition, LSDV can cause mastitis and orchitis as well as necrotic plaques on the membranes of the upper respiratory tract, the oral cavity and lungs. Several reports have previously described diagnostic methods for detecting LSDV following either experimental19 or natural infection.10 Histological examination only cannot definitively confirm LSD. For this reason, IHC was applied to detect LSD antigen and PCR to detect viral DNA. Detection of LSDV antigen was previously applied in formalin fixed paraffin embedded skin nodules from naturally infected cattle using a monoclonal antibody generated to a capripoxvirus viral core protein (ORF 057) and expressed in Escherichia coli.12
In the present study, FMD was diagnosed at El-Dakahlia province, Egypt based on clinical signs, gross and histopathology and then confirmed using RT-PCR detection. Gross and histopathology findings of FMD reported in the present study were similar to those previously mentioned by Ali et al.20 FMD molecular studies determined the variation and genetic relationship among the circulating FMDV serotypes in Egypt. This genetic variation can partially explain persistent occurrence of outbreaks even with obligatory governmental vaccination.21–23
Infectious bovine papillomatosis is a contagious disease of cattle caused by bovine papilloma viruses (BPV) characterized by the presence of variable sized warts on the skin, either localized or generalized.24 Extensive lesions lead to depreciation in the aesthetic and economic value of the animal owing to loss of body condition, hide value, increased risk of trauma, consequent wounds, hemorrhages, myiasis, necrotic dermatitis, mastitis and interference in suckling, milking and coitus.24 Cutaneous papilloma and fibropapillomas were recently described by Moharram et al.25 in El-Dakahlia province. Among all skin diseases encountered in the study conducted by Fantaye and Melake,7 wart was the fourth main skin disease with significantly higher prevalence in animals less than two years of age than the older age group. Goldschmidt and Hendrick26 found that most virus-induced papillomas were self-limiting and usually found in young animals which develop good immunity afterwards. Histopathological findings were similar to those reported by.27,28
Dermatophytosis or ring worm is an infection of keratinized tissues in domestic animals caused by one of the two genera fungi, Microsporum and Trichophyton.29,30 Dermatophytosis caused the greatest economic and human health consequence in most developed countries.31 In the study conducted by Fantaye and Melake,7 the prevalence of ring worm was significantly higher in animals less than two years of age than the older age group. Ring worm is self-limiting in healthy adult animals but wide spread and persistent in debilitated young animals. Animal susceptibility is also influenced by immunological status.10,29 The animals housed in close proximity to each other for long periods in the fattening period in the presence of infected one lead to spreading spores of fungus between animals.32 Higher incidence and the greater infection rate of ring worm were reported in winter.10 Our clinical findings were similar to those reported by El Ashmawy and Ali.33 Microscopically, epidermis showed hyperkeratosis and acanthosis and destruction of hair follicles as mentioned by Kumar et al15. Dermatophytosis can be diagnosed by direct microscopic examination of skin scraping for endothix or ectothrix fungal spores.33 Fungal hyphae can be demonstrated only by fungus stain such as PAS and Gomori's methanmine silver as reported by Kumar et al.15
LSD, FMD, warts and ring worm were diagnosed in 10 local dairy farms at El-Dakahlia province with higher susceptibility of cattle calves than buffalo calves. FMD becomes an endemic disease with higher morbidities than in other diseases. Mortalities were reported with FMD and LSD infection. Histopathology alone was not sufficient to diagnose viral infection. IHC cannot diagnose LSDV in all infected cases. To design appropriate control strategy, proper hygienic measures should be applied including: 1-combating arthropods, 2- use of sheep pox vaccine due cross reactivity between LSD virus and sheep pox virus, 3-use of PCR for detecting LSD, FMD and papilloma viral nucleic acids in skin samples.
None.
The author declares no conflict of interest exists.
None.

©2021 Awadin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.