Journal of
eISSN: 2377-4312


Research Article Volume 12 Issue 1
1Department of Dairy Chemistry, Kamdhenu University, India
2Department of Dairy Technology, Kamdhenu University, India
3Department of Dairy Engineering, Kamdhenu University, India
Correspondence: Tanmay Hazra, Assistant Professor, Department of Dairy Chemistry, Kamdhenu University, Amreli-365601, Gujarat, India, Tel +91 9541053968
Received: May 16, 2023 | Published: May 23, 2023
Citation: Mangroliya PA, Hazra T, Sindhav RG, et al. Development of bio-strip based test for ascertain proper milk pasteurization. J Dairy Vet Anim Res. 2023;12(1):61-64 DOI: 10.15406/jdvar.2023.12.00324
Alkaline phosphatase (ALP) enzyme is naturally present in raw milk which is used as a prominent indicator to determine proper pasteurization in milk. However, determination of ALP activity in milk either time-consuming or required sophisticated instrument facilities that are uncommon in India's rural dairy or collection centers. Present test is based on the ALP reaction with p-nitrophenyl phosphate to release p-nitrophenol and inorganic phosphate in the presence of water. When p-nitrophenol reacts with a certain chromogen, the strip's colour changes from purple to grey, which may be seen with the naked eye. This strip has a nine-month shelf life at room temperature.
Keywords: alkaline phosphatase, bio-strip, pasteurization
ALP, alkaline phosphatase; IDF, international Dairy Federation; LTLT, low temperature long time; HTST, high temperature short time; p-NPP, para-nitro phenyl phosphatase; SNF, solid not fat; FSSAI, food safety and standard authority of India
Milk is a rich source of both macro and micro nutrients; therefore, it is considered as complete food for all age group people.1,2 Due to perishable in nature and presence of high quality of nutrients, milk is a very ideal medium for growth and development of many pathogenic as well as non-pathogenic micro-organisms, which are important cause of spoilage of milk as well as leads to foodborne diseases.3 Heat treatment, especially Pasteurization is the most common unit-operation to eliminate pathogens from milk or dairy products. Inadequate or improper pasteurization not able to kill pathogenic micro-organisms, leads to foodborne diseases even death.3,4 Various techniques based on dye reduction tests, redox potential measurement-based test or Alkaline phosphatase (ALP) activity tests have been tried to ascertain the proper pasteurization of milk.5 Among all these tests, measuring ALP activity is considered one of the accurate and suitable method to ascertain proper pasteurization of milk.4
Alkaline phosphatase (ALP) is one of the significant endogenous enzymes; generally associated with milk fat globule membrane.6 A wide variations of Alkaline phosphatase (ALP) activities have been observed in different species of milk.7 This enzyme able to break phosphate group associated with casein and other different types of bio molecules including nucleotides, proteins, and alkaloids.6,8 It is a highly heat resistant enzyme that could be destroyed to the elimination temperature of most of the pathogenic microbes (71.6°C for 15 second).5,6 Complete elimination of ALP activity is considered as proper pasteurization of raw milk. IDF9 recommended that ALP test should be used as a marker of proper pasteurization milk and other dairy products like cream or cheese. A couple methods like spectrophotometry, colorimetric, electrochemical or fluorometric based etc. have been successfully applied for the detection and determination of ALP activity in milk.10,11 The very basic step to determine the ALP activity in milk requires incubation of milk or milk products with chromogenic or fluorogenic substrates followed by spectrophotometry, colorimetric, or fluorometric analysis.12 Hence, these methods are time consuming as well as highly trained man power and sophisticated instruments are required. Therefore, applications of these methods in in-field testing conditions especially in rural based dairy industries where trained manpower and sophisticated instruments are very rarely available.5,13 Very recently paper kit based immune-sensing assay has been developed by Mahato & Chandra,5 to detect proper pasteurization of milk and this said technology is rapid, robust and easy to handle; but fabrication of this paper strip requires highly sophisticated lab facility. Thus, a simple, portable, rural area friendly (where lab facilities are constrained) kit is a need of time to determine proper pasteurization of milk. Considering all the above stated facts, present study was aimed to develop a simple, quick and economical dry reagent strip for detection of ALP activity in milk to check the proper pasteurization of milk.
Collection of milk and heat treatment of milk
Pooled (cow and buffalo) raw milk samples were collected from local farmers of Amreli, India; in pre-cleaned and sterilized amber coloured glass bottles. Glass tubes containing 3 ml milk sample were taken and placed in precision water bath set to 63ºC, 72ºC, 100ºC Temperature. The milk is heat treated as per following condition viz: low temperature long time (LTLT, 63ºC for 30 minutes), high temperature short time (HTST, 72ºC for 15 seconds) and for boiled (100ºC for 10 minutes). The temperature and time for heat treatment monitored constantly. After, reaching the desired temperature and time, tubes were removed and placed in ice-cold water.
Preparation of chemicals
The Tris buffer obtained from Loba Chemie Pvt. Ltd. Mumbai, India. The substrate 4-Nitrophenyl disodium orthophosphate and bromocresol purple solution were obtained from S D fine-chem. Ltd. Mumbai, India.
The substrate 4-Nitrophenyl disodium orthophosphate solution (0.4mg/ml) was prepared in 1M tris buffer solution. The pH of buffer adjusted to 8.2 with hydrochloric acid (HCl).
Screening of chemicals to ascertain the pasteurization of milk based on the basis of alkaline phosphatase activity in liquid phase
One millilitre of well mixed milk was taken in test tube. The pasteurized milk was marked as controls, while raw milk considered as sample. Two millilitres of 4-Nitrophenyl disodium orthophosphate substrate solution were added to each test tubes followed by addition of 0.15 ml of bromocresol purple indicator solution. Observe the colour within two minutes.
Development of strip
3 ml of bromocresol purple dye and 10 ml of buffer substrate (pH 8.2) was poured in the clean and dry Petri plate. Cotton Cloth (Khadi cloth) pieces (1*1 cm2 size) were dipped in it for at least 5 minutes and then dried at 30º C for 2 hours in BOD incubator. After drying the strips were pasted on the inert plastic sheet (1*5 cm2 size) with non-reactive fabric glue. The strips were stored in dark air tight bottle.
Screening of chemicals to ascertain the pasteurization of milk based on the basis of alkaline phosphatase activity in liquid phase
In order to develop dry strip, we first standardize the protocol in wet chemistry-based method. However, to standardize the said test protocol in initial phase of trials different conditions of substrate were tried. The observations for these conditions are given in Table 1. On the basis of screening of suitable parameters, the substrate p-NPP (0.4mg/ml) was prepared in 1M tris buffer (pH 8.2).
|
Different concentration of substrate |
|||||
|
Conc. of substrate |
pH of substrate |
Colour change |
|||
|
|
|
Boiled Milk |
LTLT Past. Milk |
HTST Past. Milk |
Raw milk |
|
0.2mg/ml |
8.2 |
Purple |
Purple |
Purple |
Colour change to Basalt-grey in 20 min |
|
0.4mg/ml |
Purple |
Purple |
Purple |
Colour change in Basalt-grey 2 min. |
|
|
0.8mg/ml |
Purple |
Purple |
Purple |
Colour change to Basalt- grey in 10 min |
|
|
Different pH of substrate |
|||||
|
pH of substrate |
Conc. of substrate |
Colour change |
|||
|
|
|
Boiled Milk |
LTLT Past. Milk |
HTST Past. Milk |
Raw milk |
|
8.2 |
0.4mg/ml |
Purple |
Purple |
Purple |
Colour change in 2 min. (Purple to Basalt-grey) |
|
8.5 |
Purple |
Purple |
Purple |
Colour change in 5 min. (Purple to Basalt-grey) |
|
|
9.5 |
Purple |
Purple |
Purple |
Colour change in 8 min. (Purple to Basalt-grey) Intensity of colour is more |
|
|
Different quantity of substrate and sample |
|||||
|
Conc. & pH of substrate |
Substrate: Sample |
Colour change |
|||
|
|
|
Boiled Milk |
LTLT Past. Milk |
HTST Past. Milk |
Raw milk |
|
0.4mg/ml & pH 8.2 |
1:01 |
Purple |
Purple |
Purple |
Basalt grey |
|
Low colour intensity |
|||||
|
In 10 mins |
|||||
|
1:02 |
Purple |
Purple |
Purple |
Basalt grey |
|
|
Low colour intensity |
|||||
|
2:01 |
Purple |
Purple |
Purple |
Basalt grey |
|
|
High colour intensity |
|||||
|
|
|
|
In 2 min |
||
Table 1 Screening of Reagents (concentration, pH and quantity of substrate)
The developed rapid wet chemistry-based method for assessing heat treatment or proper pasteurization of milk is based on the detection of activity of ALP in milk samples. Activities of ALP in raw milk (unheated) and pasteurized milk samples (LTLT& HTST) were measured qualitatively by observing the change of colour within two minutes. It was observed (Figure 1) that raw milk from cow or buffalo exhibited activities of ALP enzymes; therefore, purple colour was changed to grey colour complex. In this test, chromogen and substrate p-NPP on reacting with ALP in milk sample changes the colour from purple to grey complex. It was observed in Figure 1A and 1B) that in pasteurized milk for both cow and buffalo, the colour were remain purple. However, in raw milk the colour was changed to Basalt grey. Also, in boiled milk the colour was remain same (purple) without any change in colour. Present observance clearly indicated that during heat treatments (LTLT, HTST or boiling) of milk, due to elimination of ALP enzyme, no colour change was observed.
Development of Strip based assay
This difference in raw and heated milk, encouraged us to develop the dry strip by immobilization of chemicals in suitable matrices. Different types of paper based (chromatographic paper, Filter paper and nylon paper) and cloth (Khadi cloth) based matrices were earlier evaluated to immobilize the chemicals. As no appreciable differentiation between raw and pasteurized could be made by using different paper matrices for designing the dry strip. So, paper matrices were not used for further development of strip. Gautam14 not able to immobilized chemicals in paper-based matrices during development dry reagent-based strip to detect ALP activity in milk. Rani15 reported that the cloth was a suitable matrix for the development of bio-strip for determining the proper pasteurization of milk.
Therefore, Khadi cloth was used as matrices for the development of this strip. All the above stated chemicals were immobilized in strip (procedure is given in material and method section). The strip was dipped in to raw and pasteurized milk. Figure 2A and 2B depicts that a clear distinction can be made between raw and heat-treated milk (boil & pasteurized milk). In presence of alkaline phosphatase in raw milk, the colour of the strip turned into Basalt-grey colour but for pasteurized milk the colour was remain purple. The response time of the strip is 2 minutes and within two minutes this strip can able to ascertain the proper pasteurization of milk. The strip has sensitivity of > 1.5 units/L; or milk having more than 1.5 units/L of ALP activity could be detected.

Figure 2 Detection of Alkaline Phosphatase activity in milk using dry reagent strip; 2a (a) Cow boiled milk, (b) Cow LTLT Past. Milk, (c) Cow HTST Past. Milk, (d) Cow raw milk; 2b (a) Buffalo boiled milk, (b) Buffalo LTLT Past. Milk, (c) Buffalo HTST Past. Milk, (d) Buffalo raw milk.
Pasteurized market milk samples (different brands) having different fats and SNF (solid not fat) were also procured from local market. Figure 3 depicts that all the ten strips are purple in colour, no colour change were observed. Hence, it indicated the proper pasteurization of milk and elimination of ALP by proper pasteurization of milk. Therefore, it could be concluded that efficacy of this strip did no effected by different fat or SNF of milk. To ruled out the effect of adulteration, on the efficacy of this strip; different adulterants were added to milk and tested the efficacy this strip. The observation depicts in Table 2. It was observed that owing of adulteration in milk the colour of the strip was remained purple for heat treated milk (pasteurization & boiling) but the colour of the unpasteurized milk changed to basalt-grey colour. Hence, it could be concluded that the efficacy of this strip did not affect by any means of adulteration in milk. This Khadi based strip was found to be stable at room temperature for up to nine months when stored in dark bottle under humidity-free conditions.
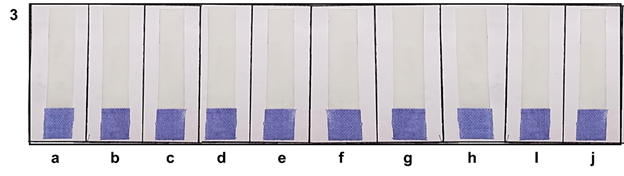
Figure 3 Detection of Alkaline Phosphatase activity in pasteurized market milk (diff. Fat and SNF) using dry reagent strip; (a) %Fat:6.0, %SNF: 9.0; (b) %Fat:1.5, %SNF: 8.5; (c) %Fat: 3.0, %SNF: 8.5; (d) %Fat: 7.0, %SNF: 9.0 (e) %Fat: 4.5, %SNF:8.5; (f) %Fat: 1.5, %SNF: 9.0; (g) %Fat: 0.5, %SNF: 8.7; (h) %Fat: 6.5, %SNF: 9.0; (i) %Fat 3.5, %SNF: 8.5; (j) %Fat: 6.0, %SNF: 9.0.
|
Adulterant |
Cow Milk |
Buffalo Milk |
||||||||
|
Colour Change |
Time Taken |
Colour Change |
Time Taken (Min.) |
|||||||
|
Boiled |
LTLT Past. Milk |
HTST |
Raw Milk |
(Min.) |
Boiled |
LTLT Past. Milk |
HTST |
Raw Milk |
||
|
Milk |
|
Past. Milk |
|
|
Milk |
|
Past. Milk |
|
|
|
|
Urea @0.2% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Sucrose @0.02% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Glucose @0.02% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Salt @0.02% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Starch @0.02% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Neutralizer @0.04% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Ammonium Salts @0.15% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Maltodextrin @0.3% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Detergent @0.02% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
|
Formaldehyde @0.006% |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Purple |
Purple |
Purple |
Basalt Grey |
2 |
Table 2 Effect of different adulterants on the dry reagent strip assay
A couple of analytical techniques have been earlier tried to ascertain proper pasteurization of milk.6 An immune based of rapid and sensitive strip protocols were earlier applied to ascertain the proper pasteurization in milk.3,5 But, for development of these kits special sophisticated laboratory and skilled man powers are required; those facilities are scare in most of the rural Indian Dairy industry. Moreover, it was also taking about 13-15 minutes to give the result. Few strip-based protocols have been developed (March, 2002) for detection of proper pasteurization in milk. These protocols require highly sophisticated instruments and specific disposable system. While, the said developed test is sensitive, easy to handle, economical and rapid strip-based protocol to ascertain the proper pasteurization of milk. Also, fabrication of bio-strips is very easy and does not require any specific training.
Traditional tests adopted by dairy industries are either expensive, time consuming require different reagents and more incubation time for reading the results.3 So rapid, in-expensive easy to adopt test kit to determine proper pasteurization of milk is a need of time, as FSSAI recommends to test pasteurization efficacy for every batch of market milk. Hence, this strip is quick, simple and easy to handle without any prior operating knowledge. Moreover, this strip can be prepared economically in any rural based dairy industry with minimum facilities.16
The present study describes the development of Khadi Cloth-based bio strip to ascertain the proper pasteurization of milk based on ALP activity in milk. The developed strip was rapid, economical, and easy to handle; therefore, readily prepared in any rural based dairy industry with minimum laboratory facilities without having any sophisticated instruments. The colour of the pasteurized milk remains purple colour but for raw milk this colour turns to basalt grey colour within two minutes in room temperature; the observation can be recorded with naked eye without having any sophisticated instruments. Shelf life of the strip is nine months when stored in dark bottle under humid free conditions.
All authors are thankful to Vice Chancellor and Director of Research, Kamdhenu University Gujarat, India for providing all facilities to carry out this research project.
Author declares there is no conflict of interest in publishing the article.
None.

©2023 Mangroliya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.