Journal of
eISSN: 2377-4312


Research Article Volume 9 Issue 6
1Department of Nutrition and Animal Husbandry, University of Veterinary Medicine and Pharmacy in Košice, Košice, Slovakia
2Department of Biology and Physiology, University of Veterinary Medicine and Pharmacy in Košice, Košice, Slovakia
Correspondence: František Zigo, University of Veterinary Medicine and Pharmacy in Košice, Department of Nutrition and Animal Husbandry, Košice, Komenského 73, 040 01, Slovakia
Received: November 25, 2020 | Published: December 28, 2020
Citation: Zig F, Ondrašovi?ová S. Control methods for reduction of mastitis in ewes caused by bacterial pathogens. J Dairy Vet Anim Res. 2020;9(6):182-187. DOI: 10.15406/jdvar.2020.09.00302
Controlling the risk factors and eliminating the causative agents of mastitis is crucial for modern dairy sheep farms. The aim of this study was to analyze during two milking seasons the effect of proposed anti-mastitis measures focused on the reduction of mammary gland infection caused by bacterial pathogens in ewes. During the study, aseptic samples of foremilk were collected from 400 ewes three times: at the beginning (in May), in the middle (in July), and at the end (in October) of the lactation season. Totally, 2211 samples were examined over two years. Cytological and bacteriological analyses of samples were performed to evaluate the relationship between the implemented anti-mastitis measures and intramammary infections (IMI). In the 682 positive samples, the most prevalent mastitis agents detected during all examinations were coagulase-negative Staphylococci (CNS), 47.5% (n = 324 udder samples) with S. chromogenes, S. epidermidis, S. warneri and S. xylosus being the most-prevalent species. Other detected species were Staphylococcus aureus (27.4%) and Streptococcus sanguinis (15.1%), isolated mainly from clinical forms of mastitis. In the first season, due to application of antibiotic treatment to positive sheep, exclusion of chronically ill individuals from the herd and consistent implementation of the hygiene rules at milking, the prevalence of IMI was reduced by 45.2% to 30.5% in the period from May to October. In the second season, after the introduction of a complex of preventive measures consisting in examination of ewes after lambing and weaning, regular maintenance of hooves, replacement of bedding, ensuring adequate nutrition, adherence to a hygienic milking programme and rejection of positive ewes that did not respond to antibiotic treatment, the incidence of mastitis was reduced from 28.0% (in May) to 17.8% (in October). A comprehensive approach is inevitable for achieving an appropriate control and prevention of mastitis in dairy farming and for the production of high quality milk with maintenance of udder health.
Keywords: Sheep, milking, coagulas negative staphylococci, hygienic programme, mastitis
The demanding requirements on the quality of sheep milk can be met in practice by application of a whole complex of hygiene and epidemiological measures set by legislative provisions with respect to conditions of its acquisition by conventional hand and progressive machine milking technology.1,2 Under real conditions of continuous operation on sheep farms, even with technologically different ways of housing and milking, many factors act simultaneously and may support the survival and spread of pathogenic microorganisms causing inflammation of the mammary gland (Figure 1). To date, over 130 different organisms have been identified as causative agents of intramammary infection (IMI), including bacteria, viruses, mycoplasma, yeasts and algae. However, 95% of infectious mastitis are caused by one of the following bacterial pathogens: Staphylococcus aureus, Mannheimia haemolytica, Streptococcus uberis, Streptococcus agalactiae, Strepococcus dysgalactiae, Escherichia coli and coagulase negative staphylococci.3,4
The mammary gland may be exposed to the mentioned pathogenic bacteria on one occasion or for a long time, but the combination of adverse external factors such as malnutrition, poor housing hygiene, faulty milking and reduced resistance of the body may result in acute or subclinical forms of IMI.4 The manifestations of inflammatory processes vary widely, as they depend on the degree of the response of the udder tissue to injury or infection. The clinical manifestations of the mammary gland inflammation as well as its further course depend on the interplay between the innate resistance and adaptive immunity of the dairy cow and the type, concentration, and virulence of udder pathogens. If the mammary gland is infected with a large number of pathogens or more germs that are virulent, and the host's defence systems are not sufficient to control the infection, an acute or chronic form of mastitis will develop.5 In sheep, the incidence and course of mastitis differs from that in dairy cows. It usually occurs suddenly, peracute or acute forms of IMI, it is difficult to manage therapeutically, and the dominant pathogens are strains of Staphylococcus spp. and haemolytic streptococci.4,6 Reducing the incidence of mastitis is difficult due to the polyethiological and multifactorial nature of the disease, therefore a successful breeder should have certain personal attributes: he must fully understand the complexities of the disease, know the principles of prevention and control, be motivated and determined, be able to motivate his employees and lastly, be able to put the comprehensive knowledge into practice. Improving the health of the mammary gland and producing quality milk can only be achieved through the application of broad-spectrum mastitis prevention and control programmes.6,7
The antimastitis measures must cover all aspects of both the external and the internal environment based on daily husbandry practices that affect the health of the ewes and their milk production. The required aspects include:
The aim of the study was to monitor the prevalence and etiology of mastitis in ewes kept on a farm with machine milking technology after the introduction of targeted preventive anti-mastitis measures during two lactation seasons.
Sheep herd and milking
The presence of bacteria in samples of sheep milk was monitored during two consecutive milking seasons on a sheep farm with machine milking technology. The farm is situated in northern Slovakia with capacity of 400 sheep of breeds Lacaune, improved Wallachian and Slovak dairy sheep. At the beginning of the season and in the summer period, approximately until the end of August, the milking frequency was twice per day, in the morning and inevening after returning from the pasture. At the end of August, in September and approximately by October the milking was performed once a day and ewes were prepared for the dry period. Throughout the milking season, the ewes are milked outdoor by means of a mobile machine parlour Farmtec. It is an in-line milking parlour with 2x12 milking stands with a food tray in between (Figures 2&3).
Examination of ewes and collection of milk
A comprehensive examination of sheep followed by milk sampling was carried out in both consecutive seasons as follows: 1st examination in May; 2nd in July and 3rd in October. The ewes were examined clinically according to Hariharan et al.,10 e.g. for swelling, presence of lesions or anatomical malformations of the udder, and milk from individual halves was evaluated by the California mastitis test (CMT). The CMT scores were 0, +, ++, and +++ for: “negative”, “weak positivity”, “positive”, and “strong positivity”, respectively.11 Teat ends were cleaned with chlorhexidine before sampling. The first streams of foremilk were discarded, and the next 10 ml of milk was collected aseptically from each udder into separate sterile vials. The samples were immediately placed into a refrigerated container with a temperature of 5º C. In the laboratory, the samples were diagnosed by cultivation and isolation of bacterial agents causing mastitis in ewes, according to the commonly accepted rules described by Malinowski et al.12 The following procedures were performed: culturing on 5% blood agar, Medium No 110 and Baird Parker agar, assessment of bacterial growth on nutrient media, Gram staining, catalase activity, coagulation of rabbit plasma, haemolysis, pigment and other. After cultivation and detection of virulence factors, bacterial colonies were identified biochemically using the STAPHY-test, STREPTO-test, or ENTERO-test and the software TNW Pro 7.0 (Erba-Lachema, CZ), with precision of detection over 90.0%.
The proposed methods of suppression of mastitis during two milking seasons in the monitored herd
Technological, hygiene and organizational conditions at obtaining sheep milk during the first season corresponded to the usual routine with regard to both milking and in the treatment of animals. The proposed preventive measures after the first examination of the herd were aimed at reducing the impact of negative factors mainly with respect to transmission and further spread of infection to healthy individuals. Antibiotic treatment of all individuals with acute mastitis and of S. aureus and S. sanguinis positive ewes was initiated. The ewes with chronic mastitis or atrophy of the secretory tissue in udder quarters after an unsuccessful treatment were rejected (Figure 4). During the milking process, post-milking teat dipping was implemented to minimize the potential for intramammary infection. The teats were immersed in an effective disinfectant solution to reduce exposure of the mammary gland to pathogenic bacteria by limiting pathogen penetration through the teat canal into the glandular tissue. During the last month of lactation, all positive sheep were subjected to a dry-off antibiotic therapy (1 syringe/gland). In the second milking season, the breeder used the results of our examinations and implemented the proposed anti-mastitis measures. These measures included supplementation of feed with mineral elements (selenium) and vitamins (vitamin E) during pregnancy. The health status of the sheep after lambing, housing of the young with their mothers, provision of quality nutrition, regular replacement of bedding in lambing areas and formation of groups according to the age of the lambs were checked regularly. Special attention was paid to the management of lamb weaning, preparation of ewes for milking, strict selection of ewes according to the results of clinical examination of the mammary gland and bacteriological examination of milk samples. Throughout the second milking season, the hygiene of milking was checked, supplemented with a regular monthly CMT check performed the animal husbandry technician, and the function of the milking machine (vacuum level, vacuum reserve per milking unit, pulsation rate and ratio) was checked, too. At the end of this second season, all sheep that required treatment because of their positivity during the season were subjected to a dry-off antibiotic therapy.
Statistical analysis
Statistical analysis was performed by means of a software Microsoft Excel 2016. Statistical differences in the severity of mastitis were calculated by Chi Square test and the level α = 0.05 was considered statistically significant.
According to Bramis et al.13 the annual incidence of clinical mastitis in ewes is generally lower than 5%, but in some herds it can climb to the level of 30 ‒ 50%. In the case of subclinical mastitis, a prevalence ranging from 5% to 30% was detected. The results of our study indicated a high prevalence of subclinical (30.6%) as well as clinical forms (14.5%) of mastitis at the beginning of the monitored period (May – the first milking season). In the second season, at the end of the monitoring period, the incidence of subclinical forms was 14.7% and that of clinical mastitis reached 5.4% (Graph 1&2). The levels of infection, despite the declining dynamics of IMI in the three examinations during the first and second seasons (Table 1), indicated a disproportionately high number of hidden subclinical forms of IMI (Graph 1) caused mainly by the CNS. Results of 682 positive samples showed that the most prevalent mastitis agents during all examinations were CNS (47.5%), Staphylococcus aureus (27.4%) and Streptococcus sanguinis (15.1%) (Table 2). Serious problems were caused by S. aureus and S. sanguinis that were isolated mainly from the clinical forms. Leitner et al.14 indicated that staphylococci are the major etiological agents of mastitis in dairy sheep flocks. Particularly, CNS and S. aureus are the bacteria most commonly isolated from the cases of subclinical and clinical mastitis.
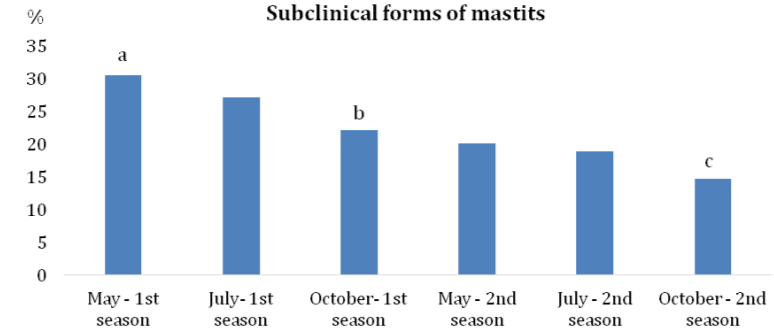
Graph 1 Prevalence of subclinical forms of mastitis during two subsequent seasons.
Note: a-cOverall values without a common superscript differ significantly at P < 0.05.
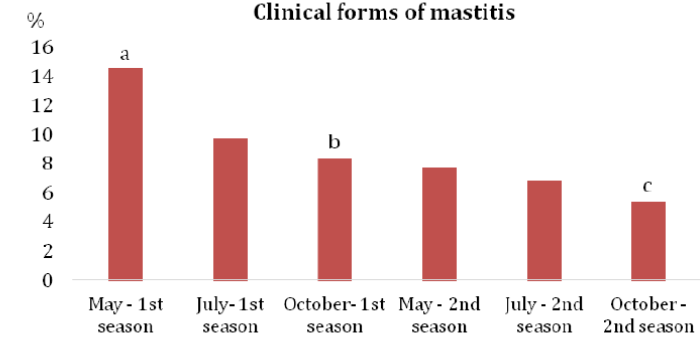
Graph 2 Prevalence of clinical forms of mastitis during two subsequent seasons.
Note: a-cOverall values without a common superscript differ significantly at P < 0.05.
|
Examination period |
Bacteriological examination of milk samples |
|||||
|
n |
Positive |
% |
Negative |
% |
||
|
1st season |
May |
385 |
174 |
45.2 |
211 |
54.8 |
|
July |
360 |
133 |
36.9 |
227 |
63.1 |
|
|
October |
348 |
106 |
30.5 |
242 |
69.5 |
|
|
2nd season |
May |
390 |
109 |
28.0 |
281 |
72.0 |
|
July |
380 |
98 |
25.7 |
282 |
74.3 |
|
|
October |
348 |
62 |
17.8 |
286 |
82.2 |
|
|
Total |
2211 |
682 |
30.8 |
1529 |
69.2 |
|
Table 1 Herd examination during two subsequent milking seasons
Note: n – number of investigated ewes
|
Herd examination time |
Positive ewes |
S. aureus |
CNS |
S. sanquinis |
Other bacteria |
|
|
n |
% |
% |
% |
% |
||
|
1st season |
May |
174 |
35.1 |
32.7 |
20.1 |
12.1 |
|
July |
133 |
32.3 |
39.1 |
19.5 |
9.0 |
|
|
October |
106 |
27.3 |
50.0 |
17.0 |
5.7 |
|
|
Total |
413 |
31.2 |
40.1 |
19.1 |
9.4 |
|
|
2nd season |
May |
109 |
34.9 |
45.0 |
11.0 |
9.1 |
|
July |
98 |
19.4 |
54.1 |
9.2 |
17.3 |
|
|
October |
62 |
16.7 |
59.4 |
13.9 |
10.0 |
|
|
Total |
269 |
23.6 |
52,8 |
11.1 |
11.5 |
|
Table 2 Prevalence of bacterial pathogens from positive milk samples
Note: CNS – coagulase negative stapylococci representing S. chromogenes, S. epidermidis, S. warneri and S. xylosus
Staphylococcus aureus causes one of the most common types of acute mastitis which often turns chronic after an unsuccessful treatment. After treatment, the IMI caused by S. aureus is usually subclinical, resulting in elevated somatic cell counts but no detectable changes in milk or the udder. The bacteria persist in mammary glands, teat canals, and teat lesions of infected ewes and are contagious. The infection spreads at milking time when S. aureus-contaminated milk from an infected gland comes in contact with an uninfected gland, and the bacteria penetrate through the teat canal. Once established, S. aureus infections do not respond well to antibiotic therapy and the infected ewes must be segregated or culled from the herd.15 The clinical forms of mastitis were treated individually, usually with therapeutically appropriate antibiotics, depending on the intensity and the extent of inflammation. First, all sheep with acute IMI were treated. Based on laboratory diagnosis of bacterial pathogens, sheep with subclinical IMI caused by S. aureus and S. sanguinis were also treated. In unsuccessfully treated individuals with an atrophied mammary gland, their separation and reassignment to the group for fattening and slaughter was recommended.
According to Bergonier and Berthelot16 and Vršková et al.17 inappropriate milking practices may have a negative impact on the sanitary status of the ewe’s udder. In general, optimization of operations needs to be combined with an efficient use of milking machine by the staff; this further requires the training of the staff on standard milking and general operating procedures. A post-milking teat disinfection introduced in the monitored sheep herd during the first and second milking seasons reduced significantly the exposure of the udder to bacterial pathogens. According to Mavrogianni et al.,18 the post-milking teat disinfection of dairy ewes is not commonly practiced in contrast to dairy cattle. It should be stressed that the appropriate sanitary status of teat dip solution can significantly reduce mammary gland contamination and transfer of bacterial pathogens to non-infected udders.
At the beginning of the second season, the breeding and preventive antimastitis measures focused on the control of ewes and weaning lambs, the treatment of positive individuals at the beginning of lactation, adherence to a milking hygiene programme supplemented with post-milking teat disinfection and regular monthly monitoring by CMT. According to Bramis et al.,13 prolificacy may have a significant impact on the incidence of mastitis in sheep. In ewes, increased incidence risk of IMI has been reported for ewes suckling two or more lambs. This could be due to teat lesions caused by teat bites during the frequent suckling events of lambs. It is therefore necessary to meet the weaning date of the lambs and to examine the mammary gland immediately before the machine milking begins. A significant effect of the introduced antimastitis measures was reflected in the reduction of the incidence of subclinical and clinical forms by 15.9% and 9.1%, respectively. Additional effect of the introduced measures was evident in the reduced incidence of bacterial pathogens, especially S. aureus from 31.2% to 23.8% and Streptococcus sanguinis from 19.1% to 11.1% at the expense of an increase in the CNS share from the original value of 40.1% to 52.8% in the second season.
The introduction and application of preventive anti-mastitis measures in the monitored sheep herd reduced the incidence of mastitis by 27% in the course of two milking seasons. During the first season, due to the consistent observation of the hygiene programme of milking and treatment of ewes, the incidence of mastitis was reduced from 45.2% to 30.5% in the period from May to October. In the second season, after the introduction of a complex of preventive measures, the incidence of mastitis was further reduced from 28.0% (in May) to 17.8% (in October). The positive effect of the introduced measures was reflected in the reduction of S. aureus and S. sanguinis by 18.4% and 6.2% respectively. These agents were isolated mainly from clinical forms of mastitis.
The abundant knowledge obtained by systematic research of this issue confirm the need to take into account the polyetiological and multifactorial character of IMI in the everyday dairy farming practice. A comprehensive approach is necessary to determine the necessary control and prevention measures that may help to dairy farmers to produce the high quality milk while maintaining the udder health. One should keep in mind that mastitis cannot be completely eliminated from the herd but can only be kept at the lowest possible level.
This study was supported by the Slovak grants APVV No. SK‐PL‐18‐0088, KEGA No. 006UVLF‐4‐2020, and VEGA No. 1‐0529‐19: The effect of environmental agents of mastitis in dairy cows and ewes on the production and degree of oxidative stress.
The authors declare that there are no conflicts of interest.

©2020 Zig, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.