Journal of
eISSN: 2377-4312


Research Article Volume 8 Issue 1
1Department of Veterinary Surgery, Anesthesiology and Radiology, Assiut University, Egypt
2Directorate of Veterinary Medicine, General Organization of Veterinary Services, Egypt
3Department of Veterinary Pathology and Clinical Pathology, Assiut University, Egypt
4Department of Statistics, Mathematics and Insurance, Assiut University, Egypt
5Department of Statistics, Virginia Polytechnic Institute and State University, United States
Correspondence: AbdelKhalek Samy AbdelKhalek, Department of Veterinary Surgery, Anesthesiology and Radiology, Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt, Tel 20 1118566358
Received: January 15, 2019 | Published: January 22, 2019
Citation: AbdelKhalek AS, Youssef HA, Ali MF, et al. An assessment of clinical, biometric, cosmetic and microscopic outcomes of four suture techniques for cutaneous closure of laparotomy wounds: an experimental study in rabbits. J Dairy Vet Anim Res. 2019;8(1):42?53. DOI: 10.15406/jdvar.2019.08.00241
The purpose of this study is to identify a suture technique that provides superior cosmetic outcomes and proper wound healing for skin closure after midline laparotomy of small animals in a rabbit model. This study also attempts to clarify the role of selected four suture patterns in wound healing, wound complications, cosmetic impacts and histopathology on the laparotomy skin wound. Twenty four female rabbits of white New Zealand and California breeds were used for this experimental study. Four suture patterns were compared for cutaneous closure of a 7–cm midline laparotomy wound, classified as; buried continuous subcuticular–intradermal (BCSID), interrupted cruciate mattress (ICM), running horizontal mattress (RHM), and far–near–near–far (FNNF). The different groups were studied in terms of clinical findings, wound and suture biometrics, cosmetic assessment, microscopic examination, and statistical analysis. RHM presented a 'very good' cosmetic grade on a 6-element scale, clinically associated with rapid successful wound healing, and no complications. BCSID was superior in cosmetic terms among the tested groups where it showed an aesthetically 'excellent' score. However, the technique was not efficient enough to prevent wound dehiscence in some cases. ICM demonstrated a 'very good' cosmetic degree but was not functional enough to prevent invasive contamination or infection in certain instances. FNNF was the inferior among all groups in regards to the cosmetic outcomes but was characterized by successful, slowly-progressive healing. Suture-to-wound length (SL: WL) ratio of all groups seemed to have a correlation with the rate of wound dehiscence as wound dehiscence is unlikely to occur if a SL: WL ratio is more than 4:1. The microscopic results proved that the RHM suture pattern was the favourable technique. RHM can be concluded as the suture technique of choice for cutaneous closure of laparotomy wounds in small animals like rabbits. It serves as a compromise of high-quality cosmesis and optimal wound healing. The assessed suture techniques can be graded in a descending order from the superior to the inferior cosmetically as BCSID>RHM>ICM>FNNF, and histopathologically as RHM>FNNF>BCSID>ICM. Clinical trials are needed to be performed to validate and reproduce the outcomes of this study on canine and feline patients.
Keywords: cosmetic surgery, suture technique, wound healing, laparotomy, histopathology
Laparotomy is a commonly performed surgical procedure in small animal practices and referral hospitals.1,2 Technical considerations when performing the operation include an appropriate patient preparation, surgical approach and wound closure.2 It is important to know whether the wound closure by continuous or interrupted suture techniques provide proper wound healing and lower complication rates, so as to allow veterinary surgeons to make an informed decision when choosing a pattern for an abdominal closure.2,3 Several suture techniques are routinely used for cutaneous closure of surgical wounds, specifically those implemented in laparotomy operations of small animals as dogs and cats.3–5 Surgeons and pet owners have various controversial attitudes on the subject of cosmetic surgery.6,7 However, the owners and breeders take special pride in their pet's appearance for dog/cat show competitions, and are deeply shocked by the poor cosmetic outcome of a given surgery, even if patient function and comfort have been restored or maintained.7 An ideal suture technique should provide accurate, quick and secure skin edge apposition for a sufficient length of time until complete healing occurs.8 Major wound complications include wound dehiscence, haemorrhage, infection, evisceration or herniation that may develop to worsen the final results.9–11 In non-complicated cutaneous wounds, an in-depth look shows that fibrotic scar deposited during skin wound healing can cause disfiguration and loss of dermal function.12 The need for rapid, aesthetic, and effective closure of the cutaneous wound has increased over recent years.9–13 Useful suture techniques improving cosmetic results include deep dermal sutures with buried knots, simple interrupted sutures, vertical mattress, horizontal mattress, subcuticular sutures, continuous over-and-over sutures and far–near–near–far pulley sutures.14 A variety of suture materials are also available to select appropriately for each wound.15 In the past, a general veterinary practice would have stocked only silk, catgut, nylon and perhaps stainless steel, but we now use a range of synthetic absorbable and non-absorbable sutures.16 The role of sutures in wound repair process is dedicated to provide haemostasis and adequate support for healing tissue. Wound healing is the reestablishment of tissue continuity that occurs through a complex process with well-orchestrated cellular and biochemical events activated at the time of injury.17,18 The process is conceptually divided into three continuous and overlapping phases; inflammation, proliferation, and maturation.18,19 There has been an assumption that wound healing is a relatively homogeneous process across species. We need to look into the presentation of wound healing in various veterinary and human surgical studies, and to the fact that almost all knowledge of cutaneous wound healing has been derived from studies on relatively few species as rodent, pig, dog, horse, and human.20 As the skin of rabbits resembles in a great extent to that of cats, an experimental study in rabbits gives pivotal results that can be more representative and mimic to those of cats.21 Many researchers in veterinary and human practice have investigated the healing of laparotomy skin wounds with regards to aesthetic impact.7,9,22 Previous studies on dogs,7 cats,11,13 rabbits,23 and rats24 have shown that the approach and design of suture technique exerts a clinical, biomechanical, physical, and histological influence on the quality of wound healing. This study aimed to compare between four suture techniques for skin closure of midline laparotomy incisions in order to achieve superior cosmetic results, effective wound closure, lowest postoperative complications, and optimal wound healing. The four suture techniques involved buried continuous subcuticular–intradermal suture pattern (BCSID), interrupted cruciate mattress suture pattern (ICM), running horizontal mattress suture pattern (RHM), and far–near–near–far suture pattern (FNNF). The different suture patterns were assessed in terms of clinical, biometric, cosmetic and microscopic outcomes of the cutaneous wound healing in a rabbit model.
Animals
Twenty four (24) clinically healthy female rabbits of white New Zealand and California breeds (8 NZ and 16 CA) whose age ranged between 63–132 days (Mean±SEM; 111±5.5days) with a body weight of 1055–2910gm (1965±97.4gm) were used for the study. Recommendations of the Guide to the Care and Use of Experimental Animals were followed to come up with the concepts of animal welfare and proper management.25 The rabbits were obtained from Poultry Farm of the Faculty of Agriculture, Assiut University (Assiut, Egypt), and clinically examined to ensure good health status and absence of any dermatological disorders.
Experimental design
The study design was approved by the Board of Veterinary Surgery Department Council and Faculty of Veterinary Medicine Council with a sample size of 20–30 animals considering animal reduction principles. The animal sample size was determined in the current study by a power calculation from a previous study on cats that has used 24 animals.11 Randomization was assigned by two participants who were blinded to the study design. The animals were chosen randomly for ear identification using a permanent marker. Randomization was carried out via a paper lottery. The animals were divided into four groups, each with two subgroups. Each group involved six rabbits; and each subgroup included three rabbits. The four groups were classified according to the skin suturing pattern. The corresponding subgroups were subdivided based on the time sequence of histopathological samples’ collection on the 10th and 17th postoperative day (POD).
Surgical operations
The surgical operations were performed by the same surgeon at the Small Animal Operation Unit, Department of Veterinary Surgery, Anaesthesiology and Radiology, Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt. Surgeries were performed under general anesthesia by IM injection of ketamine hydrochloride (Ketamine®, 50mg/ml, Sigma-Tec, Egypt) and xylazine hydrochloride (Xyla Ject®, 20 mg/ml, Adwia Co., Egypt) at the dose rate of 50mg/kg for ketamine, and 10mg/kg for xylazine. The animals were secured in a dorsal recumbency. The operation field was aseptically prepared in a routine manner at the ventral abdominal wall. A 7–cm longitudinal midline incision in the skin, subcutaneous tissue, linea alba and peritoneum was performed. In all groups, the subcutaneous tissue, abdominal muscles and peritoneum were closed in a mass closure fashion with 4 interrupted cruciate mattress sutures using absorbable monofilament 3–0 polydioxanone (PDX) (Unicryl M®, UniMed, KSA) as in Figure 1. The skin incision was sutured in the different groups by four different suture techniques. The four suture patterns involved;
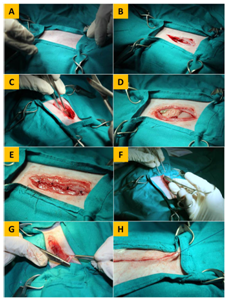
Figure 1 Plate showing the operative technique of laparotomy in the rabbit. (A) Placement of towels and clamps. (B) Incision of the skin, with dissected subcutaneous tissue. (C) Incision of the linea alba before peritoneal penetration. (D) Exploration of abdominal viscera after peritoneal penetration. (E) Suturing of the abdominal muscles with four cruciate mattress sutures. (F) Suturing of the subcutaneous tissue. (G) Insertion of the needle in the dermis for placement of the initial buried suture in BCSID closure. (H) Burial of the end suture after apposition of the skin edges prior to cutting off the suture thread in BCSID closure.
Configurations of the suture techniques were illustrated in a schematic diagram(Figure 2). The BCSID suture technique was performed with fully buried knots. The suture materials used for skin closure were;
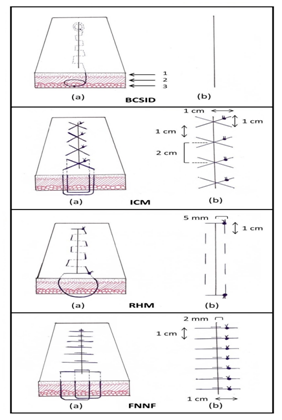
Figure 2 Schematic diagram illustrating the dimensions of each suture pattern in the skin. BCSID: buried continuous subcuticular intradermal, ICM: interrupted cruciate mattress, RHM: running horizontal mattress, FNNF: far–near–near–far, (1) epidermis, (2) dermis, (3) subcutis, (a) suture course, (b) external view.
Postoperative care
A long-acting oxytetracycline antibiotic (Alamycin LA®, 200 mg/ml, Norbrook, UK) was IM administered as a single dose following the surgery at the dose rate of 20mg/kg. A protective non-compressive abdominal bandage was applied for 6 days using sterile gauze and replaced after every wound examination post surgery. The rabbits were housed during the entire study period under the same conditions at the Experimental Animal House, Faculty of Human Medicine, Assiut University, Egypt. The animals were allowed free access to food and water ad libitum. A maintenance diet of both commercial feed pellets (Vinos®, Vinos Feed Service Co., Egypt) and fresh vegetables (carrot and parsley) were given in a natural light/dark cycle, with an ambient/warm room temperature.
Clinical assessment
The skin wounds were followed up on postoperative days (PODs) 0, 2, 4, 6, 8, 10, 12, 14, 16 and 17 for clinical findings including; scab (inflammatory exudate), haemorrhage, hyperaemia, purulent exudate, dehiscence, and swelling. Presence of sinus, fistula or incisional hernia was also inspected. Parameters for clinical assessment of wound healing were summarized in Table 1 which constitutes a modified method after Pedrajas.26 The scab color was assessed through a colorimetric index of wound healing continuum.27 Wound dehiscence percentage was calculated by means of dividing the length of dehiscence on the entire wound length per cent.28 The surgery duration of skin closure was also recorded.
|
Parameter |
Response |
|
Scab (inflammatory exudate) |
Type: serous/sero-sanguineous/sanguineous |
|
Amount: none/scant/moderate/copious |
|
|
Coloura: Colorimetric index of wound healing continuum |
|
|
Haemorrhage |
Amount: none/mild/moderate/severe |
|
Purulent exudate |
Amount: none/scant/moderate/copious |
|
Swelling (elevation) |
Degree: none/mild/moderate/severe |
|
Dehiscence |
Percentageb: WD %=(Lx100)÷E |
|
Sinus/Fistula/Hernia |
Existence: absent/present |
Table 1 Parameters for clinical assessment of wound healing: a modified method after Pedrajas
WD %, wound dehiscence percentage; L, length of dehiscence; E, entire wound length;
a Gray et al. 2010
b Hohenleutner et al. 2000
Wound and suture biometrics
Measurements of the wound length were taken on PODs 0, 5, 7, 10 and 17 for evaluation of wound contraction. The length of consumed suture in surgery was measured for calculation of the suture-to-wound length ratio (SL: WL) as described by Varshney et al.29 This calculation was carried out via a mathematical formula; consumed suture length (cSL)=preoperative suture length (pSL)–remained suture length (rSL). Then, the consumed suture length (cSL) was divided on the preoperative wound length (pWL).
Cosmetic assessment
The scoring system used for cosmetic assessment of the healing wounds was the Stony Brook Scar Evaluation Scale (SBSES) with a slight modification after Rao et al.30 Two assessors performed the cosmetic assessment; of which one assessor was aware of the different groups (subjective assessment), and one assessor was blinded to the different groups (objective assessment). The parameters of the original SBSES included width, height and colour of the scar, presence of suture/hatch marks, and overall appearance on PODs 10 and 17. Hair development in the cutaneous wounds was evaluated as a new parameter to the modified scale (mSBSES) with 0 for absence and 1 for the presence of any hair on the incision line (Table 2).
|
Scar category |
Points |
|
Width a |
|
|
>2 |
0 |
|
≤2 |
1 |
|
Height a |
|
|
Elevated / depressed |
0 |
|
Flat |
1 |
|
Colour a |
|
|
Darker than surrounding skin |
0 |
|
Same colour or lighter colour |
1 |
|
Hatch/suture marks a |
|
|
Present |
0 |
|
Absent |
1 |
|
Hair development b |
|
|
Absent |
0 |
|
Present |
1 |
|
Overall appearance a |
|
|
Poor |
0 |
|
Good |
1 |
|
Total c |
6 |
Table 2 Cosmetic scoring system of wound scars: Modified Stony Brook Scar Evaluation Scale (mSBSES)
a SBSES– Standard scale parameters (Singer et al. 2007, Rao et al. 2015)
b mSBSES–A novel parameter added by the present authors to this modified scale
c Grading system of total mSBSES mean percentage, Excellent: 80–100%; very good, 70–79%; good, 60–69%; satisfactory, 50–59%; poor, 30–49%; very poor, 0-29%;
Microscopic assessment
The histopathological specimens were taken on the 10th and 17th POD from every rabbit in each group. Fresh tissue specimens of rectangular area were collected including the operative line. They were excised carefully to avoid traumatization. The specimens were fixed in 10% neutral buffered formalin. They were dehydrated in a graded alcohol series, cleared with methyl benzoate, embedded in paraffin wax, sectioned at 4-μm thickness, and stained with hematoxylin and eosin for histopathological examination by light microscopy.31 Histopathological characterization of wound healing in the different groups was blindly assessed by the same pathologist, as described by Kiani et al.13
Statistical analysis
Repeated measures two-way analysis of variance (ANOVA) was performed to detect any overall differences between the suture techniques in terms of wound length (WL) that was measured over different time points. In this analysis, the suture technique and the time of wound healing (T) were set as fixed effects, whereas the subject (rabbits) was considered random effects. The one-way ANOVA test was used to find whether the suture techniques are statistically different in terms of suture lengths (SL) and suture-to-wound length (SL:WL) ratio. A follow up analysis was performed using Tuckey’s test for multiple comparison to find which pair of techniques are different using the 95% confidence intervals. To compare the suture techniques in cosmetic scores of mSBSES, Welch’s test was used instead of one way ANOVA. This test assumes unequal variances, which is practically better than assuming equal variances in normal ANOVA and it works well when the response variable takes a few numbers of numeric values. Differences between techniques were considered statistically significant when P<0.05. The data analyses were conducted using Minitab Statistical Package® (Version 18.1, Minitab Inc., developed at Pennsylvania State University, USA).
Clinical assessment
All wounds of BCSID group showed marked hyperaemia of the skin around the incision on POD 0. The terminal ends of all wounds were slightly elevated over the initial and terminal knots. In 1 out of 6 incisions, the wound margins at the entire length were slightly elevated over the skin surface on POD 2. Sanguineous exudate was seen predominantly from POD 2 to 4. The scab sloughed off by POD 8. Two cases out of 6 (33.3%) showed partial dehiscence (WD=33.5%) at the caudal third of incisions, where these wounds eventually healed by second intention. Hair started to grow from POD 10 until it masked the whole incision line at POD 17 (Figure 3). In ICM group, the wound margins were slightly elevated over the skin surface in all animals (Figure 3). Serosanguineous exudate was found in medium quantities confined to the wound bed and partially soiled the protective dressing on POD 2, and the subsequent scab developed from PODs 2 to 10. In 3 cases (50%) on POD 6, there was an increase in the amount of exudates that could stretch the wound margins a bit to render a small filled gap between a couple of sutures. Of such cases, one revealed scant purulent exudate. From POD 12 to 17, the other wounds were seen with variable degrees of hair growth. All wounds of RHM group were slightly elevated on POD 0 (Figure 3). They gradually relieved and eventually flattened with the surrounding intact skin by POD 12. Every single wound did not show any haemorrhage or purulent discharge. Pink wound beds as well as serous scab were characteristic findings in this group from POD 2. Scab started to slough off from POD 6, and completely fell off by POD 10. Hair growth was not noticeable in 2 out of 3 cases (66.7%). Regarding hyperaemia, the skin colour in all wounds was normal except one which was mildly hyperaemic on POD 2 and returned normal on subsequent days. The skin edges in FNNF group were slightly inverted on POD 0 (Figure 3). Minimal dehiscence (WD=10%) occurred at the middle portion of incision in one case (16.7%) on POD 2 due to animal self-mutilation. No haemorrhage was observed in any day. Three cases (50%) were hyperaemic and showed marked allergic signs on POD 4 presented with rashes, denuded or erosive scaly skin in the surrounding area which recovered and appeared normal by POD 8. The suture marks of all wounds of the group left a characteristic railway-like appearance. No sinus, fistula nor incisional hernia developed in any of the four groups. The surgery duration of skin closure lasted for 44-56 (Mean±SEM; 49.5±2.0) minutes in BCSID group, 15–18 (17.2±0.5) minutes in ICM group, 16-19 (17.5±0.6) minutes in RHM group and 15–21 (18.2±0.9) minutes in FNNF group.
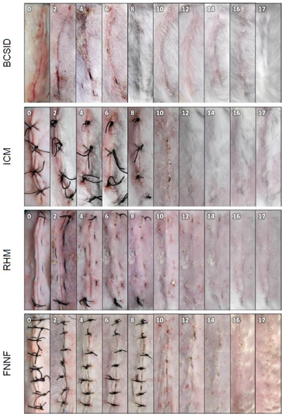
Figure 3 Representative plates showing the gross changes of successful wound healing for each suture pattern on different postoperative days (PODs 0, 2, 4, 6, 8, 10, 12, 14, 16 and 17). BCSID: buried continuous subcuticular intradermal, ICM: interrupted cruciate mattress, RHM: running horizontal mattress, FNNF: far–near–near–far. Numbers positioned in the cranial direction of the incision are referring to the postoperative day.
Wound and suture biometrics
The wound lengths (WL) of BCSID group decreased from POD 0 to POD 5 then slightly increased on POD 7 and re-decreased successively from POD 10 to POD 17 (Table 3 & Figure 4). The WLs of ICM group decreased from POD 0 to POD 10. Afterwards, the lengths returned to be around the initial WL on POD 17. Measurements of the WLs for the RHM group decreased continuously from POD 0 to POD 10 and then increased again on POD 17. All WLs of FNNF group decreased gradually from POD 0 to POD 7, slightly increased on POD 10 and declined afterwards on POD 17. The measurements for preoperative suture lengths (pSL), remained suture lengths (rSL), consumed suture lengths (cSL), preoperative wound lengths (pWL) and suture-to-wound length ratios (SL: WL) for each group were recorded in Table 4.
Group |
POD 0 (cm) |
POD 5 (cm) |
POD 7 (cm) |
POD 10 (cm) |
POD 17 (cm) |
BCSID |
5.9 ±0.4 |
4.9±0.5 |
5.2±0.5 |
4.3±0.4 |
3.7±0.6 |
ICM |
6.6±0.2 |
5.7±0.4 |
4.5 ±0.3 |
4.3±0.3 |
5.2±0.2 |
RHM |
6.6±0.2 |
5.8±0.3 |
5.1±0.3 |
5.0±0.1 |
5.8±0.2 |
FNNF |
7.0±0.0 |
5.3±0.1 |
5.1±0.2 |
5.4±0.3 |
5.2±0.1 |
Table 3 Measurements of wound lengths (WL) for each suture pattern on different postoperative days
Mean±SEM (standard error of mean); POD, postoperative day;
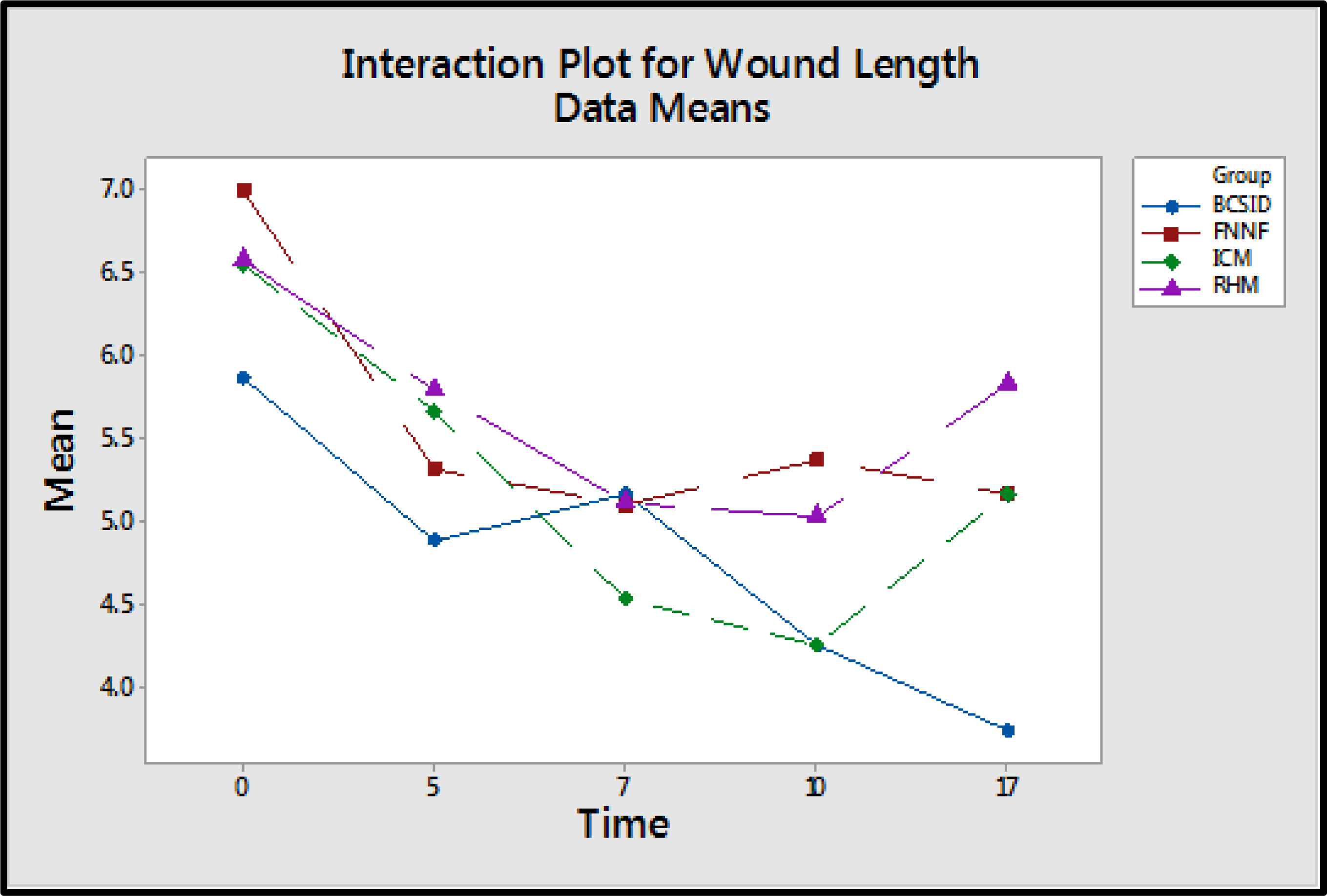
Figure 4 An interaction plot displaying a potential interaction between the suture techniques and the time of wound healing. It shows that the wound length averages of the techniques are different and the performance of the techniques changes over different postoperative days (PODs 0, 5, 7, 10 and 17).
Group |
pSL (cm) |
rSL (cm) |
cSL (cm) |
pWL (cm) |
SL:WL (ratio) |
BCSID |
75 (PDX) |
60.4±0.2 |
14.6±0.2 |
7 |
2.1:1 |
ICM |
90 (silk) |
64.9±0.2 |
25.1±0.2 |
7 |
3.6:1 |
RHM |
90 (silk) |
73.1±0.2 |
16.9±0.2 |
7 |
2.4:1 |
FNNF |
90 (silk) |
51.6±0.3 |
38.4±0.3 |
7 |
5.5:1 |
Table 4 Values for preoperative suture lengths, remained suture lengths, consumed suture lengths, preoperative wound lengths and suture-to-wound length ratios
Mean±SEM; pSL, preoperative suture length; rSL, remained suture length; cSL, consumed suture length; pWL, preoperative wound length; SL:WL, suture-to-wound length ratio; PDX, polydioxanone;
Cosmetic assessment
The wound scars of BCSID group on POD 10 had an aesthetic total score 4.5±0.7 out of 6 (where the number 6 is the optimum score of modified Stony Brook Scar Evaluation Scale). Such score of the BCSID group was graded as 'very good' as 75%. Later on POD 17, the score increased to 5.0±1.0 out of 6 (excellent, 83.3%) (Table 5 & Figure 5). The cosmetic outcome of the ICM group was initially 'poor' (mSBSES=2.8±0.9, 47.2%) on POD 10. Over time, it improved to became 'very good' (mSBSES=4.7±0.7, 77.8%) on POD 17. In RHM group, the resulted scars were 'satisfactory' with mSBSES score (3.2±0.5, 52.8%) on POD 10. Increasingly, the score had the same outcome of ICM (4.7±0.7, 77.8%) on POD 17. The FNNF group had a 'very poor' cosmetic outcome at the first time of evaluation (POD 10; mSBSES=0.5±0.3, 8.3%). On the 17th postoperative day, the aesthetic features of its scars improved but remained 'poor' looking and they did not reach a satisfactory level of cosmesis (mSBSES=2.7±0.9, 44.4%).
|
Group |
POD 10 (points) |
% a |
POD 17 (points) |
% a |
|
BCSID |
4.5±0.7 |
75 |
5.0±1.0 |
83.3 |
|
ICM |
2.8±0.9 |
47.2 |
4.7±0.7 |
77.8 |
|
RHM |
3.2±0.5 |
52.8 |
4.7±0.7 |
77.8 |
|
FNNF |
0.5±0.3 |
8.3 |
2.7±0.9 |
44.4 |
Table 5 Cosmetic scores of each suture pattern represented by mSBSES total points on PODs 10 &17
Mean ± SEM; mSBSES, Modified Stony Brook Scar Evaluation Scale; % a Percentage of mSBSES total score in relation to the maximum score of the scale (6)
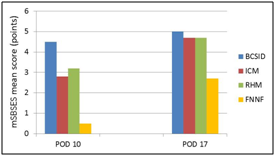
Figure 5 Chart illustrating comparative results for the cosmetic outcomes of wound scars expressed by the mean scores of mSBSES for each suture pattern on PODs 10 and 17.
Microscopic assessment
Histopathological characteristics of the skin tissue closed by the BCSID suture pattern varied in both phases of wound healing on the 10th and 17th postoperative day. On POD 10, a detectable amount of immature connective tissue was observed at the deep layers of dermis along the wound incision; afterwards it developed on POD 17 as abundant mature collagen bundles infiltrated with chronic inflammatory cells. Pseudo-healing was observed in one incision and a moderate wound gap was shown between the wound margins with keratinization. Vascular changes expressed by varying degrees of neovascularization were seen in the dermis in one case on POD 10 with haemorrhage (Figure 6A). This reaction was replaced by connective tissue infiltration of the dermis and subcutaneous tissue with a moderate to high number of monocytes, lymphocytes and plasma cells and foreign body giant cells aggregated around the suture (Figure 6B). The wounds closed by the ICM suture pattern demonstrated two cases with inverted wounds. Suppurative inflammation occurred in one of the two inverted wounds. The number of acute inflammatory cells increased in such case on POD 10 leading to pus formation at the epidermal surface and was associated with mild haemorrhage within the dermis (Figure 6C). A mild degree of chronic inflammatory cellular reaction was also observed in all wounds on POD 10 which disappeared later on POD 17. Formation of granulation tissue began to develop at the base of the wounds but the amount was scant and remained constant in both phases of the histopathological changes at the 10th and 17th day post wounding (Figure 6D). On day 10 post-surgery with RHM suture pattern, the epidermal cells began to cross over the injury and underwent partial reepithelialization. The dermis was composed primarily of unorganized dense connective tissue with thick irregular collagenous fibers with infiltration of acute inflammatory cells around the wound edges where scab was also developed on the surface to effectively seal the wound and further collapse (Figure 6E). By day 17, the wounds distinctly demonstrated complete healing as the granulation tissue fully filled the wound gap, and this was accompanied by complete reepithelialization with hyperkeratosis (Figure 6F). The wound gap on POD 10 with FNNF suture pattern showed partial healing. The granulation tissue formation was adequate to fill the entire wound space while the reepithelialization was absent (Figure 6G). On day 17, no change was detected in the amount of granulation tissue but reepithelialization was completely developed (Figure 6H). The comprehensive histopathological scores among all groups at both stages of assessment on PODs 10 and 17 were tabulated and shown in a comparative chart (Table 6).
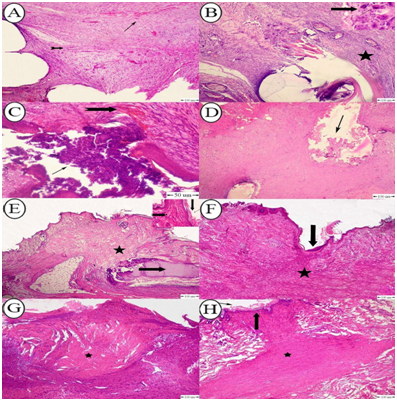
Figure 6 (A) Photomicrograph of rabbit skin with BCSID suture pattern on POD 17 showing mature connective tissue infiltrated with mild hemorrhage (arrow) and neovascularization (notched arrow); (B) Photomicrograph of rabbit skin with BCSID suture pattern on POD 10 showing connective tissue infiltrated with chronic inflammatory cells around the suture tract (star), presence of multinucleated giant cells around the suture material (arrow); (C) Photomicrograph of rabbit skin with ICM suture pattern on POD 17 showing pus formation (arrow), and mild hemorrhage in the dermis (notched arrow); (D) Photomicrograph of rabbit skin with ICM suture pattern on POD 10 showing beginning of granulation tissue formation at the base of the wound (arrow); (E) Photomicrograph of rabbit skin with RHM suture pattern on POD 10 showing immature connective tissue (star) with infiltration of acute inflammatory cells around the wound edges (notched arrow) and the epidermis underwent hyperkeratosis (down arrow) with scab formation (right arrow); (F) Photomicrograph of rabbit skin with RHM suture pattern on POD 17 showing complete healing of wound gap with complete reepithelialization (arrow), and granulation tissue formation (star); (G) Photomicrograph of rabbit skin with FNNF suture pattern on POD 10 showing complete healing with granulation tissue formation (star); (H) Photomicrograph of rabbit skin with FNNF suture pattern on POD 17 showing complete granulation tissue formation filling the entire wound gap with mature fibroblast cells (star) and reepithelialization (arrow). H&E stain. Bar=100μm.
|
Variable |
BCSID |
ICM |
RHM |
FNNF |
||||
|
POD 10 |
POD 17 |
POD 10 |
POD 17 |
POD 10 |
POD 17 |
POD 10 |
POD 17 |
|
|
Acute inflammation a |
- |
- |
++ |
++ |
+++ |
- |
++ |
- |
|
Chronic inflammation a |
++ |
++ |
+ |
- |
+ |
+++ |
- |
+++ |
|
Granulation tissue amount a |
++ |
+++ |
+ |
++ |
+++ |
+++ |
++ |
++ |
|
Granulation tissue fibroblast maturation b |
++ |
+++ |
+ |
- |
+++ |
+++ |
+ |
++ |
|
Collagen deposition a |
+ |
++ |
++ |
++ |
++ |
+++ |
+++ |
+++ |
|
Re-epithelialization c |
- |
- |
- |
- |
+ |
+++ |
- |
+++ |
|
Neovascularization d |
++ |
+ |
+ |
- |
++ |
++ |
- |
+ |
Table 6 Histopathological results showing the mean scores of wound healing assessed via Histological Scoring System (after Kiani et al)on PODs 10 and 17
a – none, +scant, ++moderate, +++abundant
b – immature, +mild maturation, ++moderate maturation, +++fully matured
c – none, +partial, ++complete but immature or thin, +++complete and mature
d – none, +up to 5 vessels per high power field (HPF), ++6 to 10 vessels per HPF, +++more than 10 vessels per HPF
Statistical analysis
One-way ANOVA test showed that the four suture techniques are not the same in suturing time (surgery duration), where P<0.05. From Tuckey’s test, it is found that the first technique (BCSID) is different from the other three techniques, FNNF, ICM, RHM, at 5% significance level. BCSID has the highest average time among the four techniques. Also, Tuckey’s test revealed that there is no significant difference between the other three methods (FNNF, ICM, RHM) and they are the same in terms of the surgery duration at 5% significance level. The assumptions of ANOVA test are checked and found that they are met. Figure 4 displays a potential interaction between the suture techniques and the time of wound healing where the wound length averages of the techniques are different, and the performance of the techniques is different over time. As a result, this interaction is included into the two-way ANOVA with repeated measures analysis which showed that the suture techniques are not the same in WL (P<0.05). It is found that BCSID and ICM have smaller average WL compared to FNNF and RHM. It is also found that there is a significance difference in suture lengths (SL), and suture-to-wound length (SL: WL) ratios (P<0.05) between different techniques. From the analysis and Tuckey results, all suture methods are different from each other. The order of these methods from least to greatest in the mean value is FNNF, ICM, RHM, and BCSID. Welch’s test results showed that cosmetic scores of mSBSES scale are significantly different between for the different methods on POD 10, where P-value is less than 0.05. Nevertheless, on POD 17, there is no significant difference (P>0.05) between the four methods.
A number of studies have been conducted to assess the effects of different suture techniques and materials on the performance of skin wound healing in laparotomy incisions.9–11,13 Demands for an aesthetic laparotomy closure, within elective or emergency procedures, are increasing in small animal practices. Scarce studies are available in the veterinary practice about the cosmetic impact of wound healing potential and the response to those approaches by which different closure methods and materials would be reliable and practical. In midline laparotomy incisions, there is a close connection between the suture technique and the wound healing process. The closure technique plays a fundamental role in this process and primarily affects both early and late wound complications.24,32 Potential complications encountered in cutaneous laparotomy closure were reported in previous studies. Examples of these problems included wound dehiscence, haemorrhage, swelling, inflammation, infection, sinus formation or incisional herniation.11,33,34 The current study attempted to investigate the challenges occurring in laparotomy skin wound healing with a special focus on the aesthetic and clinical outcomes. Four suture techniques were compared after reviewing available literatures and recommendations of each author’s preferred method. The suture patterns were selected based on their prospective cosmetic significance. Clinical findings of this study proved that the favourable technique among tested groups for cutaneous closure of laparotomy wounds was RHM suture pattern as it revealed a compromise of the most desired clinical, cosmetic and histopathological results. Although BCSID provided superior cosmetic outcomes on both PODs 10 and 17, the technique failed to counteract the postoperative factors which led to dehiscence in 33.3% of cases. Even though, the dehisced wounds eventually healed within 17days postoperatively by mixed intention healing. These findings are in accordance with those of Ghag et al.,35 where the highest number of wound complications occurred in the subcuticular group (46.7% of cases). It had also grade-III infection as a sequel of partial dehiscence. However, Gurusamy et al.,36 reported that superficial wound dehiscence may be reduced by using continuous subcuticular sutures. This difference might depend on whether the sutures were absorbable or non-absorbable, because most of these wound dehiscences were reported in two recent trials in which the continuous skin suture was by absorbable subcuticular sutures.37 Subcuticular and intradermal suture techniques are commonly used in human plastic surgery.38 In veterinary research, subcuticular patterns were also cosmetically favourable in canine,9 feline,38 and caprine skin closure.22 In one study on bovine skin, cruciate mattress suture pattern yielded good cosmetic appearance with fewer suture marks.39 In a range of studies on human facial skin, RHM suture pattern was concluded as a favourable alternative to traditional simple continuous suture pattern, with smoother and flatter scar than the conventional method.40 FNNF pattern is considered as a combination of tension and appositional suture pattern. The far component takes up the tension while the near component holds skin margins in apposition. The suture can be applied loosely without tightening to avoid inversion at the incision line.41 It is known that the more apposition is executed on a wound closure, the more cosmetic results will be achieved.42 We took advantage of the appositional property of the near component of FNNF to assess the cosmetic effect of this pattern. FNNF was recently studied in recent researches on laparotomy in human subjects with regard to its efficacy and complications.43,44 It was concluded that wound dehiscence or infection resulting from this suture pattern might be the least or none. In the present study, BCSID demonstrated elevation induced by the buried knot which appears big sized due to the four nodes of the knot; this was in consistence with Smeak34 and Chupeco et al.38 Additional security was needed since PDX was a monofilament thread. RHM is known for a characteristic feature of eversion which progresses by flattening over time. Everted wound edges tend to heal with flat scars, whereas an initially flat closure may become indented following wound contraction.40 We sought a suture technique that would provide more eversion than a simple running or running subcuticular suture and could be more rapidly performed than interrupted vertical mattress sutures. ICM was also associated with an everting pattern as its structure has cruciate limbs naturally presenting a geometrical influence of force. In the current study, owing to the lengthy line of the incision (7cm), this might affect the inversion of edges. The shadow shown in FNNF on POD 0 indicated an oblique rather than a perpendicular angle taken during photography of such wound; however there was no raised appearance at real situation. FNNF-associated wound inversion was obvious in days 2 up to 17. In harmony with Adams et al.,42 it was believed that FNNF has tendency to produce some level of both eversion and inversion of wound edges simultaneously; as an initial eversion promotes a more even-surfaced scar by counteracting the downward pull of wound edges during the healing process. The influence of using two different suture materials for skin closure might play a role on the macroscopic and microscopic healing performance. The current study used polydioxanone for skin closure in BCSID group. The use of an absorbable monofilament suture such as PDX in a continuous subcuticular pattern with a buried knot was recommended by Flecknell.45 In few preliminary trials conducted prior to this study to test PDX perforating all skin layers, it was found that PDX yielded scab and scar when attempted on full-thickness skin. A pre-informed idea of this finding might introduce some level of bias in the experimental design or protocol. However, PDX has been previously proven as a good choice when used intradermally in consistence with Perret-Gentil8 and Flecknell.45 Pavletic stated that routine closure of a laparotomy incision or a hernia repair would necessarily require the selection of nonabsorbable or very slowly absorbed suture material that would retain tensile strength well beyond the normal range of healing in a healthy patient.7 Kladakis indicated that PDX loses 14% of tensile strength by 14days, 30% by 42days and is completely absorbed by 180days.15 As a matter of fact, Cotran et al. reported that the quantitative increase in collagen ends in 10days and the cutaneous wound is moderately healed at this time. By 14 to 16days, normal wound healing in the rabbit is accomplished.46 For the aforementioned reasons, PDX and silk sutures were used in the present study. Silk sutures may not be used for skin closure in small animal practices in the USA. Where cosmetic results are important, closure and prolonged apposition of wounds and avoidance of irritants will produce the favourable result. Therefore, it was advisable to use the smallest inert monofilament suture materials such as nylon or polypropylene; to avoid skin sutures; to close subcuticularly whenever possible; and to secure close apposition of skin edges using a topical skin adhesive.8 In the UK, a survey was conducted on current practices and influences on the choice of suture material, pattern and size used in commonly performed procedures in small animal practices. For skin closure, the most common suture material used by UK veterinarians was non-absorbable multifilament. Short-acting monofilament was most commonly chosen for subcutaneous closure while long-acting monofilament was most commonly chosen for linea alba. A simple continuous pattern was the most commonly used for subcutaneous and linea alba closure overall. The cost-effective considerations had also an influence on the selection of the suture pattern, material and size.47 In Australia, the twisted coated products were much more popular among skin sutures than nylon in packets or cassettes. In the opinion of the reporters, this reflects habitual usage rather than superior properties. Simple interrupted was the most popular skin suture pattern, followed closely by horizontal mattress. Few Australian veterinarians used other suture patterns.48 In Egypt, simple interrupted suture is the most fundamental technique of wound closure used in cutaneous surgery. It possesses greater tensile strength, and has less potential to cause wound oedema and impaired circulation. Simple continuous is used in tissues that are elastic and are not subject to a lot of tension. The horizontal mattress helps in minimizing the wound tension, closing the dead space, and facilitating wound edge eversion. It is both an interrupted and a continuous pattern. Despite the sophistication of current suture materials and techniques, closing a wound still involves the same basic procedure based on Halsted’s principles. Development of good suture technique requires a deep understanding of the rational mechanics involved in suturing. Many experienced surgeons, even those who have performed surgeries for many years, have developed poor surgical technique. Suture coding plays an important role in the infrastructure for surgical sutures as every suture pattern has its own code that helps in the structure and method of application.49 Common complications that occur after abdominal wound closure include, but not restricted to, wound dehiscence, infection, seroma, haemorrhage, peritonitis, or adhesion.26 One of the reasons that may be countable for wound dehiscence in BCSID group is the abdominal pressure.28 The load exerted by the viscera on the skin wound may impair the wound healing. The dehisced portions were mostly situated at the caudal half of the incision line. This may be due to settlement of the large intestine in such region. The load exerted by the caudally-located intestine was thought to be greater than the cranially-situated stomach. The healing process was still in primitive stages on the first few days so the injured tissue could not bear the abdominal pressure. Therefore, the BCSID was weak enough to cause dehiscence; this was supported by Adams et al.42 and Harari.50
In the present study, cutaneous wound dehiscence of some cases (33.3%) in BCSID group might be attributed to other factors such as the insufficient tissue of that thin skin which was held by the subcuticular sutures, as well as the postoperative traumatization that might occur to the sutured skin. The SC tissue was engaged in BCSID through alternative tissue bites in the beginning, middle and end of the incision. Potential influence of inadequate SC sutures in the middle of the incision (where the tension will be the most) might have a reflection on the BCSID findings of the current study due to the difference in the amount of tissue incorporated. In small young rabbits, the SC tissue is highly fragile in nature and can be easily torn;51 that may also have a possible influence on wound dehiscence. Smeak34 suggests that this complication can be avoided by suturing the external rectus fascia with healthy bites (at least 0.5cm) without incorporating any SC tissue within these bites; suture size and knot technique must be correct; and closure pattern can be both simple interrupted or continuous. The author also prefers continuous pattern in most cases with the exception if the abdominal wall edges are unhealthy so it is better to place interrupted sutures. If tension relief is needed, it is advisable to pick up only the hypodermis during SC closure (as SC wound reaction is most likely caused by tissue devitalization when taking large SC tissue bites and pulling sutures tight which rips the tissue from its blood supply). Such wound reaction that is observed is due to the body’s response in removing the devitalized fatty tissue during the debridement phase. Bohling et al. clarified that extensive debridement of SC tissue may delay wound healing particularly in felines. A higher risk for wound infection may also accompany extensive removal of SC tissues in canines.52
In the present study, wound infection was a postoperative complication when ICM was used. The infection was detected in 33.3% of cases. The tension force of the suture threads in this technique was in an oblique direction to the incision line. The effect of tension was possibly lower than the other techniques in which the tension was perpendicular to the incision line. After the knots were tied, each mattress loop was crossed and partially overlapped; leading to tissue misconfiguration.29 Another reason explains why the ICM wounds were susceptible to infection is the dimensions of the suture pattern. The sutured tissues were held together only at the suture points. In the spaces between suture points, the immediate tensile strength might be effectively zero. The distance between two successive stitches at the intersection point of x-strands was 2cm. If compared to an interrupted horizontal mattress suture, such distance was two-fold greater. This created a passage between the wound margins that paved the way for wound contamination and invasion of microorganisms, as well as excessive fluid leakage which could stretch the wound margins. This interpretation was in accordance with Fried and Walsh.53 Silk sutures may be questionable for aseptic closure in situations when it represents a predisposing factor for surgical site infection8 since silk is composed of natural proteins with a braided structure. However, a combination with antibacterials can prevent the invasion of bacteria and reduce the probability of infection.54 In the present study, the antibacterial activity was induced and sustained by the use of a systemic long-acting broad-spectrum antibiotic (Oxytetracycline LA). The blood levels of the antibiotic persisted for at least five days as prescribed by the manufacturer. To our knowledge, we believe that silk is a smooth suture material acceptable for the delicate lapine skin. Silk is affordable, easily manipulated, and will not interfere with wound healing if handled carefully with a combined antibacterial agent. In the intracutaneous groups (ICM, RHM, FNNF), silk sutures resulted in an overall 83.3% normal closure rate in the rabbit’s skin. With exception for BCSID, the present study revealed no significant difference between RHM, ICM and FNNF groups in terms of the duration of skin suturing. The time lasted ~18 minutes (average) in RHM, ICM and FNNF groups due to ease handling of these techniques where closure of wounds was percutaneous passing through all layers of the skin. In contrast, Homvises55 found some problems with RHM; of which, it was time-consuming that required ~15 minutes extra time. This result was attributed to the length of sternal wound in such study. BCSID suture pattern in the current study required longer time (~50 minutes) for skin closure as high accuracy was needed to pass the suture thread through the subcutis and the dermis of the rabbit's delicate skin. In small animals, most previous studies were concerned with wound biometrics in case of second intention healing.33 The current study compared changes in first intention healing. The wound length plot showed a descending slope into shortest lengths in BCSID and FNNF groups on POD 5. This gradual shortage of WL may return to wound contraction as described by Fossum56 & Williams57 who reported that the 'pull theory' is the mechanism where fibroblasts and myofibroblasts give motive power for contraction and hence a shortage in WL. Contraction proceeds at a rate of ~ 0.6 to 0.8mm/day and it stops when the wound edges meet each other, or when tension from the skin around the wound is equal to or greater than the contractile forces created by myofibroblasts, or when there is insufficiency of these cells. In ICM and RHM groups, the WL plot was terminated with an ascending slope toward the preoperative WL. This may be due to synthesis of collagen in the skin with an aim to regain standard tensile strength of the newly formed tissue.58
In the present study, the suture-to-wound length ratio in BCSID, ICM, RHM and FNNF groups was 2.1:1, 3.6:1, 2.4:1 and 5.5:1 respectively. These results appear to correlate with the incidence of wound dehiscence. It was proven in previous studies that a SL: WL ratio more than 4:1 for laparotomy closure could reduce WD.59 However, dehiscence did not occur with RHM group of which the ratio was 2.4:1. This may be attributed to the suture material implanted in the full thickness of the skin with an equal tension applied on the wound edges that led to abundance in the remained suture and shortage in the consumed suture. Cosmetic assessments in the current study revealed that the BCSID suture pattern was the superior technique among all groups as it provided the highly cosmetic appearance of wounds on both PODs 10 and 17. Nevertheless, they showed more aesthetic outcomes on the 17th day with the highest mSBSES score. This was consistent with Sylvestre et al.9 who concluded that the buried continuous subcuticular suture pattern may influence a better cosmetic appearance on a cutaneous closure of canine ovariohysterectomy at the time of recheck visit. If in cosmetic order, the second cosmetic rank was for RHM, and the third was for ICM, while FNNF technique came at the last as it gave worst cosmetic results. In plastic surgery, the Patient and Observer Scar Assessment Scale (POSAS), and Vancouver Scar Scale are widely used for scar assessment in human patients, but it is difficult to decide which scale can be used typically for each scar condition and treatment method. The assessment of newly developed surgical scars was most commonly performed by the POSAS.60 Since the patient is involved in the POSAS assessment; this scale cannot be applied to those speechless creatures, the rabbits, but perhaps via the sponsorship of the animal owner. Clinical scar assessment lacks a standardized methodology and a systematic approach. Therefore, studies continue to lack consensus regarding the most appropriate and applicable evaluation instrument. Fearmonti et al.61 discussed the quantitative and qualitative measurement modalities that are needed to evaluate and monitor scars. They provided a review on available clinical tools and current assessment scales used to subjectively and objectively characterize scars. The authors emphasized that an optimal, universal scar scoring system is necessary to be constructed in order to better characterize, understand and treat pathologic scarring. The Stony Brook Scar Evaluation Scale (SBSES) was proposed by Singer et al.62 and its reliability was acceptable. It is a 5-element ordinal wound evaluation scale developed to measure short-term cosmetic outcome of wounds in 5 to 10 days after injury up to the time of suture removal. This scale incorporates assessments of individual attributes with a binary response (1 or 0) for each, as well as overall appearance, to yield a score ranging from 0 (worst) to 5 (best). The SBSES has only recently been suggested for use in research, as it was designed to measure short-term rather than long-term wound outcomes.61 In the current study, we innovated a new parameter added to our modified 6-element SBSES with 0 for absence and 1 for presence of any hair development on the incision line. The novel parameter acted as an indicator for optimal cutaneous healing in the rabbit where the skin retains its physiological functions with developing hair follicles and its adnexa. The mSBSES cosmetic scores in the current study were consistent with the clinical and microscopic outcomes. The degree of cosmesis in case of RHM group was low and undesirable on POD 10 due to the presence of suture marks at that time. By day 17, the technique gave an enhanced outcome of cosmesis as these marks faded away over time. These results were in accordance to Moody et al.40 who stated that RHM provides good wound eversion, reduce wound tension, and promote haemostasis. It also has a less constrictive effect because the wound edge pressure is applied only unilaterally which is evenly distributed across the entire wound line. Moreover, RHM is a continuous pattern, thus limiting the stress on the wound edges. A punctate scar (i.e. suture holes) was seen in FNNF group. Dunn58 explained that this occurs when the skin sutures are not placed with excessive tension and are removed at an early time. The forces that create either crosshatching or suture marks will remain after suture removal. Histopathological findings of the present study were consistent with such explanations where the FNNF group showed notable great amounts of mature collagen fibers. This result was in agreement with Yang et al.63 who stated that the main histological components of the skin scar are thick epidermis and dermis with tough collagenous fibers. Microscopic examination of BCSID group revealed formation of an epidermal bridge (stratum corneum) over the incision while the dermis did not develop and presented with a moderate gap. However, the wound appeared normally healed by the naked eye at the same time. Unexpected adverse results can occasionally occur in cats. Of these, Bohling33 reported such condition and called the phenomenon 'pseudo-healing'. The sutured skin incision might seem like normally healed at the time of suture removal but after that time, when the animal exerts a normal physiological stress on the closure, such as a cat jumps up from a chair, complete dehiscence occurs. In contrast, integral skin closure was evident in the RHM group on day 10, with partial reepithelialization and scab formation. By day 17, the wounds notably showed complete healing as the granulation tissue entirely filled the wound gap with complete reepithelialization and hyperkeratosis. Cotran et al. highlighted that the quantitative increase in collagen ends in 10 days and the wound is moderately healed at this time. By 14 to 16days, normal wound healing in the rabbit is complete; this was harmonic with Gourley and Gregory.64 Likewise, such changes occurred in FNNF group, but reepithelialization was absent on day 10. However, the wounds afterwards underwent complete reepithelialization by day 17. In our study, the overall histopathological findings confirmed that the RHM suture technique was superior to other groups in terms of the wound healing scoring system of Kiani et al.13 The RHM was associated with successful wound healing on both phases of evaluation on day 10 and 17. The dermal tissues showed acute followed by chronic inflammation, complete reepithelialization, full maturation of fibroblasts, and abundant formation of collagen bundles at the end of the study on the 17th postoperative day. Potential limitation of the present study might lay in the differences in the type of suture material used in BCSID group (PDX) and the other three groups (Silk). It was presumed that BCSID could provide favourable outcomes in all terms of the assessment on the account of other tested techniques. However, the overall study outcomes showed unexpected results supporting for RHM. Short-term wound monitoring may impose a restriction for expecting later cosmetic impacts, as a longer time would be required for wound examination so as to ascertain no further downsides might develop. The lack of standardized modalities of scar assessment scales, and the subjective/objective nature of these scales might have a different response to the parameters upon clinical trials. Reliability of cosmetic scores is context and content specific, and further testing required to translate them in a heterogeneous setting (different models, raters, and techniques). Future studies also need to be conducted to implement an equal approach for the aesthetic suture patterns using a uniform material and a standardized scale, hence to validate the appropriate use of these suture techniques in clinical practice.
The running horizontal mattress (RHM) suture technique may be considered the favourable suture pattern of choice for closure of the laparotomy skin wound in small animals such as rabbits. The technique was associated with an upper-second grade of 'very good' cosmetic outcomes, and rapid successful wound healing without any complication. This suture pattern serves as a compromise of high-quality cosmesis and optimal wound healing. The assessed suture techniques can be cosmetically graded in a descending order from the superior to inferior as:
In terms of microscopic assessments, they can be histologically graded as:
The study was self-funded by the first author, and received no specific grants from public funding bodies or research institutions.
Authors express many thanks to Mr. Samy ElAriny and Mrs. Huda Fares (Donators, Animal Welfare Society, Assiut, Egypt) for their generous financial contributions. The partner donators had no role in the design of the study or collection, analysis, and interpretation of data or in writing the manuscript. Acknowledgements extend to Ms. Jennifer Giannoccaro (Instructor, Department of English Language, American Institute for Training and Education, Assiut, Egypt) for proofreading of the research article.
Authors declare there are no conflicts of interest.

©2019 AbdelKhalek, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.