Journal of
eISSN: 2377-4312


Research Article Volume 7 Issue 3
Department of Production Animal Clinical Sciences, Norwegian University of Life Sciences, Norway
Correspondence: Maien Munthe-Kaas, Department of Production Animal Clinical Sciences, Norwegian University of Life Sciences (NMBU), Ullevalsveien 72, 0454 Oslo, Norway, Tel 47 41450790
Received: February 26, 2018 | Published: May 9, 2018
Citation: Kaas MM, Cohen LM, Framstad T. Acute phase response and hematology in pigs after cryptorchidism or inguinal hernia surgery. J Dairy Vet Anim Res. 2018;7(3):73-77. DOI: 10.15406/jdvar.2018.07.00193
Invasive surgery is a strain to the body, and many pigs undergo abdominal surgery in field practice. An observational study was performed to investigate changes in the composition of serum proteins, including acute phase proteins, and white blood cells after cryptorchidism or inguinal hernia surgery. Blood samples were collected one day before surgery and one, three, six and fourteen days after surgery. The study showed an average increase of 45.52 mg/L in C-reactive protein (CRP) day one after surgery compared to the day before surgery (p<0.0001). CRP levels were normalized on day fourteen post-surgery. On day three post-surgery, α- and β-globulins were significantly higher than before surgery. Albumin was significantly decreased on day six (p<0.01) post-surgery. On day fourteen post-surgery, total protein, α-, β- and γ-globulins were 3.45 g/L (p<0.01), 0.94 g/L (p<0.05), 1.14 g/L (p<0.001) and 2.97 g/L (p<0.0001), respectively, higher than the levels pre-surgery. There was an increase in the numbers of white blood cells from the day before surgery until the sixth day after surgery (p<0.05) and an increase in neutrophil granulocytes (NG) from the day before surgery until the day after surgery (p<0.05). The NG number stayed significantly increased throughout the test period. The average percentage amount of NG increased from 39.53 to 47.36 from the day before surgery until the third day after surgery (p<0.01), and the percentage amount of lymphocytes decreased accordingly (p<0.05). There was also an increase in the concentration of eosinophil granulocytes from the day before to the day after surgery (p<0.05). The study demonstrated how surgical procedures leads to an increase in the acute phase protein CRP, and α-, β- and γ-Glob, reduction in Alb, and changes in the WBC profile in the days following surgery.
Keywords: pig, CRP, serum proteins, acute phase, white blood cells, surgery
Many pigs undergo surgery under variable conditions in field practice. Today it is possible to immunologically castrate cryptorchidic pigs as an alternative to surgery. Therefore, it is of interest to enlighten the degree of stress upon the animals considering the overall benefit of the procedure. One can according to Norwegian legislation both surgically and immunologically castrate pigs from the same herd1 and immunological castration may also be used on cryptorchidic individuals. Several articles have been published on acute phase response in pigs in relation to infections,2 the impact of corticosteroids3 and following injections of turpentine4,5 and lipopolysaccharides.6 A study describing the acute phase reaction in pigs following thorax surgery was published in 2016.7 Some studies claim that a laparotomy procedure leads to a stronger acute phase and immune response than less invasive methods such as laparoscopy8 or natural orifice transluminal endoscopic surgery (NOTES),9 but a general view of the immediate changes in CRP, serum proteins or the white blood cell count in pigs following abdominal surgery is lacking in published literature. A pilot study made at the Norwegian School of Veterinary Science in 2009 measured C-reactive protein (CRP), haptoglobin and fibrinogen in 40 pigs post-surgery (Tore Framstad, personal communication). In 1998 the reference intervals for clinical biochemistry and hematology in anemic and healthy pigs at weaning was published,10 the acute phase proteins or serum electrophoresis were, however, not included in that study. Hematologic and biochemical reference intervals have been published for Norwegian grower pigs11 and for Ontario nursing pigs12 in 2010 and 2017 respectively. The aim of this study was to investigate the response of different clinical-chemical and hematological parameters in pigs after surgery for cryptorchidism or inguinal hernia operations. Changes in acute phase proteins and white blood cells (WBC) from the day before surgery until two weeks post-surgery were registered.
Animals and sample collection
This observational study involved blood sampling of pigs operated for teaching purposes at Norwegian University of Life Sciences. The procedures, sampling and surgical methods are used in clinical veterinary practice, adhere to widely recognized and standardized procedures and did not, at the time, have to be granted by the Norwegian Animal Research Authority.13,14 The animals included in this study were weaned male pigs from 8-29 kg, which were cryptorchidic or had an inguinal hernia. The surgeries were performed by veterinary students at Department of Production Animal Clinical Sciences at Norwegian University of Life Sciences in the period of January to April 2014. Students operating were performing the procedure for the first or second time. Each pig was identified in the database by the use of a journal number and ear tag number, both given at the clinic. Thirty-two pigs were registered in the database in total, including 30 cryptorchidic and 2 with inguinal hernia. Blood samples were collected from each pig the day before they underwent surgery. These samples were used as control samples for samples collected on day 1, day 3, day 6 and day 14 post surgery. All blood samples were collected from V. jugularis externa on pigs in dorsal recumbency.15 The pigs were also examined clinically and weighed on the days of blood sampling and surgery. At every sampling there was collected blood in one 4 mL tube containing ethylenediamine tetraacetic acid (EDTA) (Vacuette® K2E K2EDTA) for haematologic analysis, and one 4 mL tube without additives (Vacuette® Z Seru, Clot Activator) for serum protein electrophoresis (SPEP) and CRP. Because of some registrations missing and culling of some individuals before the study was completed, the sample population (n) for the different parameters is not constant throughout the study.
Sample analysis
The blood samples were analysed for CRP in an Advia® 1800 Clinical Chemistry System (Siemens Healthcare Diagnostics, Siemens AG Germany), hematology in an Advia® 2120 Haematology System using Advia Multi-Species software (Siemens Healthcare Diagnostics, Siemens AG Germany), and serum protein electrophoresis was executed in a Sebia Capillaries™ 2 (Sebia, Norcross GA, USA) at the Central Laboratory, Norwegian University of Life Sciences.
Surgery
The surgical technique used is described in Nor Veterinærtidsskrift in 2002.16 All the pigs were starved for at least 12 hours prior to surgery. Preoperatively they were sedated with 8 mg/kg azaperone (Stresnil®) intramuscularly (IM) and given 2 mg/kg flunixin meglumine (Finadyne®) IM. They were locally anesthetized with 20 mg/mL lidocaine with 36 μg/mL adrenaline (Lidokel-adrenalin®) in the epidural space, volume by Strande’s formula.17 After the procedure they were all given trimethoprim/sulfadiazine (Tribrissen® 80/400 mg/mL) IM. If considered necessary based on clinical examination, they were given flunixin meglumine (Finadyne®) IM the day after surgery.
Statistical analysis
Statistical analysis was performed in JMP® Pro 13.0.0 and graphic illustrations were made in Microsoft® Excel. Descriptive statistics are presented as means, standard deviations and 95%-confidence intervals. Initially the levels of CRP, TP, Alb, α-Glob, β-Glob, γ-Glob, WBC, NG, NG(%), LC, LC(%), MC, EG and BG (see abbreviations) on the different days of sampling were compared separately to each other by a one-way ANOVA. At each time point, they were specifically compared to the control samples post-hoc by using the Dunnett multiple-comparison test. P-values less than 0.05 were considered significant.
CRP and serum protein electrophoresis (SPEP)
The mean concentration of CRP in the control samples was 24.93 mg/L (Table 1). The largest change in CRP levels was from the day before until the day after surgery, when it showed an average increase of 45.52 mg/L (p<0.0001) (Figure 1). Day 3 post-surgery the levels of α-Glob (p<0.05) and β-Glob (p<0.01) were elevated compared to the control samples. The largest mean change in α-Glob was an increase of 1.07 g/L 6 days post-surgery (p<0.01). On day 14 post-surgery α-Glob (p<0.05) was still significantly elevated compared to levels pre-surgery. β-Glob and γ-Glob displayed their largest increase in relation to the control sample levels at day 14. The mean increase was 1.14 g/L (p<0.001) and 3.14 g/L (p<0.0001) respectively. The greatest measured change in Alb was seen at day 6 (p<0.01) post-surgery, with a decrease of -2.18 g/L compared to the control samples. There was no visible change in TP until day 14 post-surgery, when there was an average increase of 3.45 g/L (p<0.01) (Figure 2) (Table 1).
Haematology
In the concentration of white blood cells (WBC) there was seen an increase on day 6, 4.14x109/L (p<0.05) higher than the control samples (Figure 3). The level of neutrophil granulocytes (NG) was increased at day 1 (p<0.05), day 3 (p<0.01) and day 6 (p<0.01). The greatest increase was seen on day 3, when the average level was 3.48x109/L higher than the control samples. The increase in NG concentration was reflected in a significant increase also in the percentage amount of NG out of the total WBC count, from a share of 39.53 % the day before surgery to 47.36 % on the third day after surgery (p<0.01) (Table 2). From the day before surgery until the third day after, there was a decrease in the mean percentage amount of LC out of the total WBC count (p<0.05), from 51.63 % the day before surgery to 44.24 % the third after surgery. The level of eosinophil granulocytes (EG) measured the greatest change from the control sample levels day 1 after surgery, with an average increase of 0.21x109/L (p<0.05) (Figure 4).
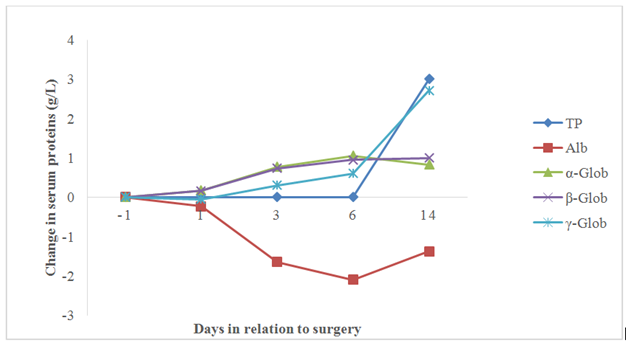
Figure 2 2 Mean change in levels of serum proteins, including total protein (TP), albumin (Alb), α-globulin (α-Glob), β-globulin (β-Glob), γ-globulin (γ-Glob) from the day before surgery until 14 days after surgery.
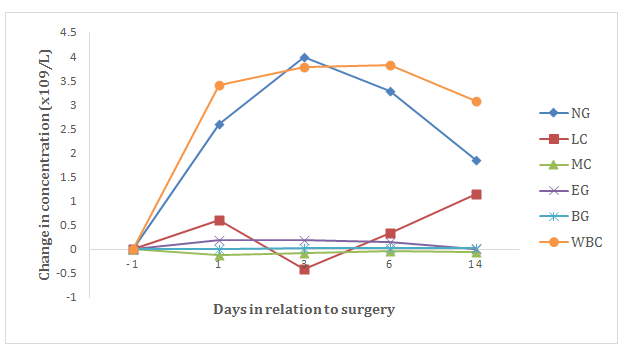
Figure 3 Mean change in concentration of white blood cells (WBC), including neutrophil granulocytes (NG), lymphocytes (LC), monocytes (MC), eosinophil granulocytes (EG) and basophil granulocytes (BG) from the day before surgery until 14 days after surgery.
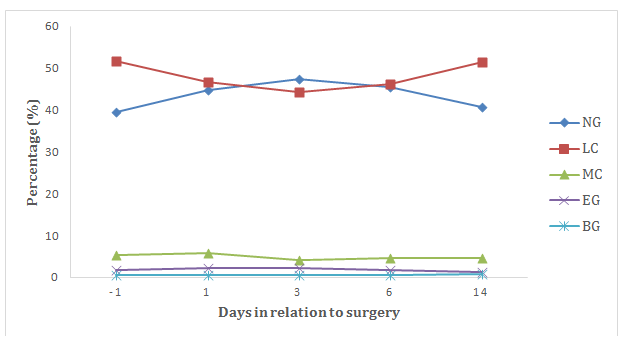
Figure 4 Mean change in percentage amount (%) of neutrophil granulocytes (NG), lymphocytes (LC), monocytes (MC), eosinophil granulocytes (EG) and basophil granulocytes (BG) from the day before surgery until 14 days after surgery.
|
Population (n) |
CRP |
TP |
Alb |
α-Glob |
β-Glob |
γ-Glob |
Day -1 |
32 |
24.93±19.75 |
47.6±3.1 |
22.04±3.03 |
10.60±1.00 |
10.03±0.90 |
4.93±1.54 |
Day 1 |
31 |
70.45****±31.24 (58.99-81.91) |
47.7±3.4 |
21.95±3.13 |
10.83±1.02 |
10.18±0.96 |
4.75±1.48 |
Day 3 |
27 |
49.76**±38.15 (34.67-64.85) |
48.0±4.1 |
20.40±3.09 |
11.46*±1.27 |
10.91**±1.23 |
5.21±1.29 |
Day 6 |
30 |
50.50**±33.88 (37.85-63.15) |
48.2±2.8 |
19.87**±2.37 |
11.67**±1.30 |
11.05***±1.01 |
5.61±1.89 |
Day 14 |
24 |
30.49±17.91 |
51.0**±3.9 |
20.45±1.94 |
11.55* ±1.17 |
11.16***±1.10 |
7.89****±2.56 |
Table 1 Mean values±standard deviation (95% confidence intervals) of CRP and serum proteins in pigs before and after surgery
Mean values±standard deviation (95% confidence intervals) of C-reactive protein (CRP) and serum proteins, including total protein (TP), albumin (Alb), α-globulin (α-Glob), β-globulin (β-Glob) and γ-globulin (γ-Glob) on the day before surgery and day 1, 3, 6 and 14 after surgery.
The superscripts *, **, *** and **** show significant differences from values measured the day before surgery at significance levels of <0.05, <0.01, <0.001 and <0.0001, respectively.
|
Population (n) |
WBC |
NG |
NG |
LC |
LC |
MC |
EG |
BG |
Day-1 |
32 |
20.30±6.42 (17.98-22.61) |
7.89±2.80 |
39.53±7.80 |
10.60±4.14 (9.11-12.09) |
51.63±7.72 (48.85-54.42) |
1.13±0.64 (0.90-1.37) |
0.33±0.19 |
0.13±0.09 (0.10-0.16) |
Day 1 |
30 |
23.95±6.33 |
10.61*±3.35 (9.36-11.86) |
44.82±9.91 |
11.30±4.41 (9.66-12.95) |
46.68±9.11 |
1.03±0.65 |
0.52*±0.33 |
0.14±0.10 (0.10-0.18) |
Day 3 |
24 |
23.47±7.94 (20.11-26.82) |
11.38**±5.83 (8.92-13.84) |
47.36**±11.03 (42.70-52.02) |
10.16±3.57 (8.65-11.67) |
44.24*±10.87 |
1.00±0.45 |
0.51±0.35 |
0.15±0.09 (0.11-0.19) |
Day 6 |
30 |
24.44*±5.68 (22.32-26.56) |
11.28**±3.88 (9.84-12.73) |
45.48*±9.33 |
11.14±2.78 |
46.29±9.31 |
1.13±0.45 |
0.45±0.32 |
0.15±0.07 (0.13-0.18) |
Day 14 |
24 |
23.68±5.27 (21.46-25.91) |
9.67±3.19 |
40.61±8.91 |
12.12±3.28 |
51.35±8.76 |
1.10±0.41 |
0.31±0.21 (0.23-0.40) |
0.16±0.09 (0.12-0.20) |
Table 2 Concentration ± standard deviation (95% confidence interval) of white blood cells (WBC) in pigs before and after surgery
Concentration ± standard deviation (95% confidence interval) of white blood cells (WBC), including neutrophil granulocytes (NG), lymphocytes (LC), monocytes (MC), eosinophil granulocytes (EG) and basophil granulocytes (BG) and the percentage amount of NG and LC on the day before surgery and day 1, 3, 6 and 14 after surgery.
The superscripts * and ** show significant differences from values measured the day before surgery at significance levels of <0.05 and <0.01, respectively.
Acute phase response and serum proteins
CRP is viewed as one of the most important acute phase proteins in pigs.18 Among its functions are to activate the complement cascade by binding to the pathogen and to decrease the negative effects of inflammation by stimulating the production of anti-inflammatory cytokines and interacting with leucocytes.19 The serum concentrations of CRP and serum proteins may vary with age. However, to our knowledge, no studies have described the normal development of CRP or serum proteins in a growing pig over time. In healthy 43 days old female piglets (n=54), it has been measured an average concentration in CRP of 8.35 mg/L, with a variation of up to 16.8 mg/L.3 In this trial the average CRP on the day before surgery was higher (24.93 mg/L), which can be due to a few extreme values on the initial sampling which were not removed from the data set. The average measured in the control samples in this study did, however, coincide with the average CRP level found in the pilot study from 2009 (Tore Framstad, personal communication). In the 24-48 hours following stimuli, e.g. aseptic inflammation or eventually an infection, the CRP concentration will increase, and can increase fast from <5 mg/L to >100 mg/L.18 The level of CRP in blood is linked to the severity of the condition and to tissue damage,20 but can also increase due to stress.3 The highest level of CRP in this study was measured approximately 24 hours after surgery (70.45 mg/L), while it had decreased markedly by day 3. The mean concentration of CRP when at its highest did not exceed 100 mg/L (Table 1). There was no blood sampling at 48 hours post-surgery, so a possible peak on this day would not be detected.
Alb is the most abundant protein in serum. During inflammatory processes, several other proteins will increase in concentration, and as a result, the colloid osmotic pressure rises. This in turn down regulates the production of Alb in the liver,21 and causes a gradual decrease in Alb concentration during an acute phase response. Hence, Alb is considered a negative acute phase protein.18 Albumin is a smaller molecule than the other serum proteins, and has a great ability to escape from the blood stream.22 Albumin can be lost via the kidneys, intestines or to body cavities, and the production will decrease if there is not enough available substrate.23 In consequence the concentration of Alb in serum can decrease as a result of one or a combination of these factors. In this study there was proven a gradual decrease in Alb concentration in the days following surgery, with the lowest measured level six days post-surgery (Table 1). This is probably due to a down regulation in synthesis during the acute phase response, but can also be linked to increased fluid loss to the interstitial in and around the surgical wound, and possibly an increased metabolic demand for amino acids for heat production. It is also possible that the pigs had a reduced feed uptake following the surgery, and therefore did not meet the demand for substrates to the Alb synthesis. α-Glob include among other acute phase proteins, for example serum amyloid-A and haptoglobin, and will therefore increase early in the inflammation process.23 In the pigs that underwent surgery there was proven a significant elevation in α-Glob from 3 days after surgery until 14 days after surgery, and the highest measured level was at day 6.
β-Glob include, among other molecules, complement factors C3 and C4,23 which are considered as acute phase proteins,24 but some immunoglobulin’s will also be separated in the β-Glob fraction (β2) during serum protein electrophoresis (SPEP).23 Some sources claim that CRP will end up between the β- and γ-fractions under SPEP in horses23,25 and in the γ-fraction in dogs,23 but it is not certain where it deposits in pigs’ serum. In this study there was proven a significant increase in the β-fraction from day 3 and throughout the test period, with the highest measured level on day 14. If CRP deposits in the β-fraction in pigs, together with the complement factors C3 and C4, that can explain the early elevation of β-Glob. Fibrinogen is also an acute phase protein that deposits in the β-fraction during SPEP, but it is spent during the clotting of the blood, and is not a part of the serum used in the analysis for this study. During chronic inflammation, of more than one week’s duration, the production of immunoglobulins is increased.23 The occurrence of immunoglobulin’s in the β-fraction can explain the observed peak in β-Glob concentration on day 14.
γ-Glob are mainly comprised of immunoglobulins (IgA, IgM, IgE and IgG) produced by exposure of foreign antigens to LC.18 The increase in γ-Glob concentration therefore arises further into the inflammatory process than the changes in α-, β-Glob, CRP and albumin. In this study γ-Glob levels were not significantly elevated until day 14. TP include all the proteins in serum, meaning Alb, α-, β- and γ-Glob. In this study the TP level was normal on the first 3 samplings after surgery, when the albumin decreased and the α- and β-Glob levels increased, and these changes leveled each other out. Not until day 14 was there an elevated level of TP. At this point β- and γ-Glob were at their highest measured level, and α-Glob was still elevated, while Alb no longer was significantly decreased.
Haematology
Healthy pigs of this age normally have a lymphocytic cellular profile,10 meaning that WBCs are dominated by LC. During acute inflammation the demand for NG raises. They are an important part of the immediate immune response, and are recruited from the bone marrow via cytokines released from mononuclear cells distributed locally at the site of inflammation. One can often see a left shift in the NGs, caused by a higher requirement of cells than the bone marrow can meet, leading to a release of immature NGs.26 It is proven that the release of adrenalin and cortisol, for instance during stress, causes an elevation in leucocytes, mainly NG, in blood. However, if there is presence of a left shift, the increase in NG is due to inflammation.26 A possible source of error in the results is stress caused by handling during sampling. The sampling was performed according to a standardized procedure; therefore the stress the animals experienced is likely to be generalisable. When an inflammation becomes chronic, the character of the response changes and will involve hyperplasia of LC. The concentration of LC in blood will normally not increase, as this process is limited to lymphatic tissue.26
The control samples showed that the pigs had a lymphocytic cell profile prior to surgery (Table 2) and that the cell profile changed in the days following the procedure. Day 3 post-surgery the pigs displayed a neutrophilic cell profile, and at day 6 the percentage of LC was again greater than that of the NG. The concentration of NG was elevated from day 1 until day 6 after surgery, while the concentration of LC showed no significant change. The day after surgery there was a significant increase in concentration of EG. This is a nonspecific response that can be linked to parasite infestation and allergies, but also lesions that produce substances which attract EG.26 This study was performed at a teaching facility where the surgeons were untrained. This affects the generalization of the results because skilled surgeons would probably spend less time in surgery and perhaps make smaller incisions, which are the factors we expect to influence the studied responses.
This study showed that cryptorchidism and inguinal hernia surgeries lead to an acute phase- and immune response in pigs. In pigs undergoing surgery the acute phase protein CRP, and α-, β- and γ-Glob increased, Alb was reduced, and the WBC profile changed in the days post-surgery compared to pre-surgery.
The authors wish to thank all the personnel working in the stables at the Department of Production Animal Clinical Sciences for their contributions to this study. The clinical procedures and sampling of the animals were conducted in accordance to the current Norwegian Animal Welfare legislation Act and the EUs Directive 2010/63 on the use of animals for scientific purposes.
No financial interests or conflict of interests exist.

©2018 Kaas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.