Journal of
eISSN: 2377-4312


Case Report Volume 13 Issue 1
Teacher of the Veterinary Medicine Course. Universidade Nove de Julho (UNINOVE), Brazil
Correspondence: Paolo Ruggero Errante, teacher of the Veterinary Medicine Course, New University of July (UNINOVE), Campus Memorial, Avenida Dr. Adolpho Pinto, 109, Barra Funda, São Paulo, SP, Brasil
Received: December 25, 2023 | Published: January 12, 2024
Citation: Errante PR. A case report of association between canine iatrogenic hyperadrenocorticism and hypothyroidism. J Dairy Vet Anim Res. 2024;13(1):12-14 DOI: 10.15406/jdvar.2024.13.00340
The canine hypothyroidism corresponds to a hormonal disorder, mainly caused by destruction of the thyroid gland, followed by idiopathic thyroid degeneration or idiopathic follicular atrophy, leading to a low production of the hormones thyroxine (T4) and triiodothyronine (T3). The canine iatrogenic hyperadrenocorticism consists of a disorder caused by the prolonged use of corticosteroids for therapeutic purposes, in most cases in allergic processes, such as canine atopy. In this case report, a twelve-year-old female Schnauzer was presented to the clinical facility with signs of apathy, polyphagia and non-pruritic bilateral symmetric alopecia that extended to the tip of the tail but sparing the head and limbs. Other clinical manifestations included polyuria, polydipsia, and bilateral keratoconjunctivitis sicca. The animal was submitted to the blood count, T4, T3, TSH dosage, dexamethasone suppression test, biochemical tests, and imaging tests. Exam results demonstrate the presence of hyperadrenocorticism and hypothyroidism. Since the use of corticosteroids in canine allergic diseases can lead to the development of hyperadrenocorticism, and hypothyroidism corresponds to the most common endocrinopathy in dogs, it is essential that the clinical veterinarian is able to identify these diseases early.
Keywords: dogs, skin, allergic disease, hypothyroidism, iatrogenic hyperadrenocorticism
Hypothyroidism corresponds to the most common endocrinopathy in dogs, causing a decrease in the production of thyroxine (T4) and triiodothyronine (T3) by the thyroid gland.1 The main cause of canine hypothyroidism is primary in nature, occurring in up to 95% of cases due to the destruction of thyroid glandular tissue due to autoimmune disease, idiopathic follicular atrophy, thyroidectomy, or thyroid tumors.2
Secondary hypothyroidism is responsible for 5% of cases, because of decreased TSH secretion by the pituitary gland due to the presence of pituitary tumors, pituitary malformations, and isolated Thyroid-stimulating hormone (TSH) deficiency.1 Tertiary hypothyroidism is caused by a deficiency in the release of TRH produced by the hypothalamus, due to malformations, neoplasms, abscesses, or inflammation of the hypothalamus.2 Canine hypothyroidism is commonly reported in middle-aged animals, between 4 and 10 years old, castrated and of both sexes.1,3
The iatrogenic hyperadrenocorticism is caused by excessive administration of glucocorticoids, and generally affects middle-aged to elderly dogs. Excessive use of cortisol interferes with hepatic gluconeogenesis, decreased tissue glucose utilization, lipolysis, and immune response. The most common signs in dogs affected by the disease are polyuria, polydipsia, polyphagia, respiratory panting, abdominal muscle weakness, alopecia, and skin hyperpigmentation.2,4
In December 2023, a twelve-year-old, female, neutered and vaccinated dog, Schnauzer, was attended. The owner reported that the animal represented apathetic behavior, and spent most of the day sleeping, with reluctant to walk. He also reported that the dog had polyphagia, polydipsia, polyuria and progressive blindness. According to the owner, the dog had an allergic skin condition since first years of life, always treated with corticosteroids. On the day of the consultation, he had no reaction to sound stimuli and was unable to move. During inspection, the dog presented an alopecic area that covered the dorsal region of the trunk up to the insertion of the tail, and that extended to the distal end. In addition, presented an ocular condition of bilateral keratoconjunctivitis sicca and right corneal ulcer (Figure 1).
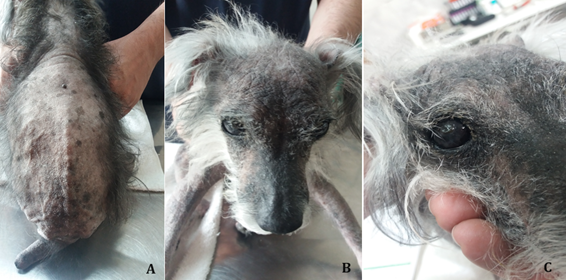
Figure 1 A. Large alopecic area that covers the dorsal region from the body to the insertion of the tail. Tail with a “rat tail” appearance. B. Bilateral keratoconjunctivitis sicca. C. Right corneal ulcer.
A complete blood count, measurement of alanine aminotransferase (ALT), alkaline phosphatase (AF), glucose, triglycerides, cholesterol, thyroxine (free T4), TSH, dexamethasone suppression test, type I urine, abdominal ultrasound and doppler echocardiography were performed.
In the blood count, a smaller number of erythrocytes (5.4 million/mm3; normal values=5.7-7.4 million/mm3), increase in the number of leukocytes (22,4 thousand/mm3; normal values=6.0-16, 0 mil/mm3) and small decrease in hematocrit (37,1%; normal values=38.0-47.0%) was observed.
The dosages of ALT (128 U/L; normal values=9-92 U/L), FA (350; normal values=10-155 U/L), glucose (125 mg/dL; normal values=60-118 mg/ dL), cholesterol (287 mg/dL; normal values=135-270 mg/dL), triglycerides (192,0; normal values=32,0 a 125,0 mg/dL) and TSH by chemiluminescence (1.10 ng/mL; normal values=0.05-0.40 ng/mL) were elevated. On the other hand, serum levels of free T4 by dialysis (0.52 ng/dL; normal values=0.80-3.50 ng/dL) were below for canine specie. In the dexamethasone suppression test, the basal cortisol value was 4,53 (normal values= 1,0 a 4,6 μg/dL), and the cortisol value 8 hours after dexamethasone was 1,86 (normal value below 0,90 μg/dL).
Type I urinalysis was performed by collecting urine through a urethral probe, and the biochemical examination showed the absence of bilirubin, glucose, ketone bodies and occult blood. In sedimentoscopy, erythrocytes (1; normal values=1-2/field), leukocytes (20-30; normal values=1-2/field) and many bacteria (normal values=absent) were observed.
Abdominal ultrasound revealed a decrease in the size of adrenal glands (Figure 2) and hepatosplenomegaly compatible with hepatic steatosis (Figure 3).
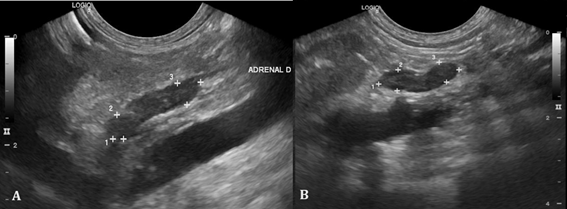
Figure 2 Ultrasound of adrenal glands. A. Right adrenal gland measuring 1.44 x 0.46 x 0.43cm (length x height of cranial pole x height of caudal pole). B. Left adrenal gland measuring 1.12 x 0.36 x 0.33cm (length x height of cranial pole x height of caudal pole).
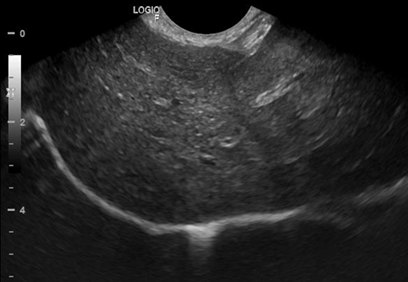
Figure 3 Ultrasound of liver. Liver with enlarged dimensions, rounded edges, high echogenicity and coarse echotexture compatible with liver disease/fatty infiltration.
During auscultation, the dog had a heart rate of 141 beats per minute (normal values = 60-160 beats/minute). The Doppler echocardiogram and color flow mapping showed the presence of moderate mitral valve insufficiency (Figure 4).
The hypothyroidism corresponds to the most common endocrinopathy in dogs, especially in older animals, being more prevalent in large and castrated dogs.3,5 In this case report, the patient was a small, neutered dog. Dogs with hypothyroidism show clinical signs of lethargy, intolerance and reluctance to exercise, and weight gain without an increase in appetite. Disease progression leads to obesity, lethargy and dermatological changes.1,2 The dog in this case report was apathetic, ate a lot and spent most of the day without moving. Most dogs with hypothyroidism present cutaneous manifestations such as bilateral and symmetrical alopecia affecting the body, ventral thorax and tail. They also present skin peeling, dry or oily seborrhea, superficial pyoderma, hyperkeratosis, hyperpigmentation, ceruminous otitis and myxedema.1 The dog reported in this case presented hyperkeratosis and hyperpigmentation of the skin, and a large alopecic area from the dorsal region of the body to the insertion of the tail, which extended to its tip, giving a “rat tail” appearance. Tail alopecia, called “rat tail” is a common dermatological sign of this disease.6
Other clinical signs include abnormalities of central nervous, gastrintestinal and cardiovascular system.7–9 The dog in this case report had bilateral keratoconjunctivitis sicca and right corneal ulcer.
Serum TSH measurement is the most important test for diagnosing hypothyroidism, since increased serum levels are confirmatory, regardless of T3 and T4 values.10 When measuring thyroid hormones, the measurement of free T4 is more reliable in maintaining its concentrations even if there are variations in the release and metabolism of total T3 and T4 or in the concentration of plasma proteins.2,10,11 In the dog under study, TSH values by chemiluminescence were elevated and serum levels of free T4 by dialysis were below normal values for the canine species.
Through the dexamethasone suppression test,2 it was possible to confirm that the dog also had hyperadrenocorticism, which justified the signs of polyphagia, polyuria and polydipsia.
Another finding after chronic exogenous administration of corticosteroids is the progressive atrophy of the adrenal gland12 caused by the suppression of CRH (Corticotropin Releasing Hormone) produced by the hypothalamus, with a consequent decrease in the plasma concentration of ACTH (Adrenocorcotrophic Hormone),2,4 according to seen on abdominal ultrasound.
The thyroid hormones influence the catabolism of adipose tissue and regulate the synthesis and degradation of cholesterol. In addition, stimulation of lipolysis by glucocorticoids causes elevations in blood lipid and cholesterol concentrations. Dogs with hypothyroidism and hyperadrenocorticism present obesity and hypercholesterolemia.1,13 However, other diseases such as diabetes mellitus can also cause an increase in serum cholesterol levels.14 In this case study, the dog had increased cholesterol and triglycerides levels with a diagnosis of hypothyroidism and hyperadrenocorticism.
A small proportion of dogs with hypothyroidism have moderate non-regenerative anemia,1,6 and dogs with hypothyroidism and hyperadrenocorticism also have a greater susceptibility to the development of infections,15 as seen in this case report.
However, in cases of canine hyperadrenocorticism, the blood count shows erythrocytosis and leukocytosis due to neutrophilia, eosinopenia, lymphopenia and monocytosis.2 In this case report, leukocytosis by neutrophilia was observed associated with infections, but not lymphopenia and monocytosis. Increases in serum levels of FA and ALT, cholesterol and triglycerides, and accumulation of lipids in the liver are described in dogs with hypothyroidism16 and hyperadrenocorticism.2
Bradycardia is a common finding in dogs with hypothyroidism, since thyroid hormones exert inotropic and chronotropic effects on the heart.17 However, bradycardia was not detected in the dog in this case report. Abnormalities found on the electrocardiogram of dogs with hypothyroidism include sinus bradycardia, weak apical beat, low QRS voltage, and inverted T waves.18 Dogs with hyperadrenocorticism present systemic arterial hypertension, thickening of the interventricular septum and concentric hypertrophy of the left ventricle with development of left heart failure.19–21 However, in the dog under study, only Doppler echocardiogram was performed, and only moderate mitral insufficiency observed.
The hypothyroidism and hyperadrenocorticism corresponds to the most common endocrine disorder observed in older dogs. Therefore, the veterinary clinician must be attentive to the clinical manifestations of these diseases, requiring laboratory and imaging tests to precoce diagnose these diseases and their complications.
None.
Author declares there are no conflicts of interests.
None.

©2024 Errante. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.