Journal of
eISSN: 2377-4312


Research Article Volume 7 Issue 3
Central University Laboratory, Tamil Nadu Veterinary and Animal Sciences University, India
Correspondence: Kumaragurubaran Karthik, Assistant Professor, Central University Laboratory, Madahavarm Milk Colony, Tamil Nadu Veterinary and Animal Sciences University, Chennai-51
Received: March 01, 2018 | Published: May 11, 2018
Citation: Karthik k, Bharathi R, Mahaprabhu R, et al. Chronic respiratory disease outbreak in an organized native chicken farm. J Dairy Vet Anim Res. 2018;7(3):79-82. DOI: 10.15406/jdvar.2018.07.00194
Indian poultry industry is moving with rapid strides in the global market but disease outbreaks cause a major setback to this huge industry. Chronic respiratory disease (CRD) caused by Mycoplasma gallispeticum (MG) is one among the important poultry disease affecting the growth of the industry. This current communication presents the outbreak of CRD among native chicken breeds in an organized farm of India. Post mortem investigation was carried out in the farm as mortality was reported to be 5% and morbidity was 50%. Samples namely trachea, lung, air sac and infra orbital sinus were collected for identifying the causative agent and were subjected to bacteriological and virological detection tests. Samples were found positive for MG by conventional bacteriological method while other bacteriological and virological agents were found negative. Isolates on further characterization using Mycoplasma specific PCR and MG specific PCR revealed that the isolates belong to MG and proved the involvement of the bacterium in causing the disease. Histopathology of the samples also showed the presence of necrotic material and infiltration of lymphocytes. Based on gross pathology, histopathology, isolation and molecular characterization showed that the disease was CRD caused by MG. Thus this reports warrants need for further insights into the clear picture on the status of CRD among native chicken breeds of India so as to carve out a better prevention measure.
Keywords: chronic respiratory disease, native chicken, Mycoplasma gallisepticum, India, PCR, investigation
The poultry industry is growing at a rapid phase worldwide in the recent years to meet the global demand for good quality protein.1,2 This rapid increase in production is projected to move further in various parts of the world including India.3 Indian poultry industry is among the top producers of egg and meat production in the world but poultry population is not constant among the states of India. Andhra Pradesh, Telangana, Tamil Nadu leads the country’s poultry production followed by Maharashtra, Punjab and West Bengal.4 This profitable industry has also witnessed the threats caused by pathogens leading to huge mortality of the birds. Among the reported diseases of poultry, chronic respiratory disease (CRD) is one of the important diseases as it can cause higher morbidity thus decreasing the production. CRD is reported from several parts of the world in both poultry layer and broiler stocks. Mycoplasma gallisepticum (MG), a cell wall less bacteria is responsible for CRD which is characterized by coughing, respiratory rales, nasal discharge, infra orbital sinusitis and air sacculitis. The disease has been recorded in layers and breeders causing embryo mortality and also drops in egg production.5 All age group chickens and turkeys are susceptible to MG but young birds are considered to be affected severely than old birds.6 Both horizontal and vertical transmission has been documented in MG infection.7 In India sero diagnosis of MG has been reported at various time frames from various states. Earlier studies show 58.18% sero positivity in Wayanad district of Kerala, India while 53.40% sero positivity was recorded in Namakkal district of Tamil Nadu, India. Recent sero prevalence study conducted in seven states of India showed an overall prevalence of 32.06%, of which highest prevalence was noted in Telangana (50%) while Karnataka state had lowest prevalence (20%).8 Sero prevalence studies are mainly carried out in India and isolation, molecular characterization studies are less. In order to meet the high demand of poultry industry exotic, genetically improved poultry breeds are developed. Major drawback of these improved breeds is their lower resistance to disease resistance compared to indigenous or native breeds.4 To the best of our knowledge reports regarding isolation, molecular and histopathological confirmation of MG in Indian native chicken breeds are inadequate. The present study documents clinical signs, postmortem and histopathology lesions followed by isolation and molecular confirmation of MG in an organized native poultry farm in Tamil Nadu state, India.
Clinical picture and sample collection
An organized farm with a flock strength of 400 native birds of various age groups was reported to have respiratory problems like nasal discharge, swelling of the infra orbital sinus and respiratory rales. Mortality 5% and morbidity of 50% was reported. Postmortem was carried out for four dead birds with similar clinical signs at Central University Laboratory, Tamil Nadu Veterinary and Animal Sciences University, Chennai, India. Gross lesions like sinusitis, conjunctivitis, tracheitis with yellow cheesy material in trachea, air sacculitis with cheesy material and pneumonia were noted (Figure 1). Heart blood swab, tracheal swab, air sac swab, infra orbital sinus swab, liver surface swab were collected in ice. Trachea, lungs, infra orbital sinus and air sac were collected in ice and also in 10% formalin separately for isolation and histopathology respectively. Samples were also subjected to diagnose infectious laryngotracheitis (ILT) by PCR so as to rule out the exact cause of the disease.
Isolation and identification
Samples were processed for bacteriological and mycoplasma isolation by standard procedures. For Mycoplasma isolation, samples were initially enriched in PPLO broth (Himedia) supplemented with 15% heat inactivated horse serum (Invitrogen), 10% yeast extract (HiMedia), 10% glucose (HiMedia), 1% NAD (HiMedia), 2.5% thallium acetate (HiMedia), benzyl penicillin sodium salt (100000 IU) and 0.5% phenol red, for a period of 3-7 days or till the media changes colour from red to yellow at 37°C under 5% CO2 tension. Broth cultures are sub cultured on PPLO agar plate and kept at 37°C under 5% CO2 tension for 5-10 days.
Molecular identification
To extract the DNA following protocol was followed: 1mL of broth cultures were taken in microfuge tubes and centrifuged at 10,000 rpm for 5 min. The pellet obtained was washed twice with phosphate buffered saline (PBS) and the final pellet was dissolved in 100µL nuclease free water (NFW) followed by heating the tube to 95°C for 10min, immediate cooling at -20°C for 5 min. Then the tube with mixture was centrifuged at 10,000 rpm for 5min and the supernatant was used as DNA for polymerase chain reaction (PCR). Initially, the extracted DNA was screened by PCR using Mycoplasma group specific primers (GPO- 1 [5’-ACT CCT ACG GGA GGC AGC AGT A-3’] and MGSO [5’-TGC ACC ATC TGT CAC TCT GTT AAC CTC -3’]) as per Kuppeveld et al.9 Positive samples were screened for MG using the primers MG-14F: GAG CTA ATC TGT AAA GTT GGT C and MG-13R: GCT TCC TTG CGG TTA GCA AC reported by Lauerman.10 Agarose (1.5%) gel electrophoresis was carried out for the PCR products and the gel was visualized under Bio Rad Gel Doc XR+ system.
Histopathology
All samples in 10% formalin were embedded in paraffin and sections of 5μm thicknesses were made from the paraffin blocks which were stained with hematoxyline and eosin.11
After 2 days of incubation at 37°C with 5% CO2 tension, colour change in the PPLO broth was noticed and this was sub cultured further on PPLO agar plates. Fried egg colonies appeared on PPLO plates from trachea, lung, air sac and infra orbital sinus swab samples after 3 days of incubation. The samples were also processed for identification of other bacterial and viral agents but they were found negative for other agents. Extracted DNA from cultures of trachea, lung, air sac and infra orbital sinus swab samples were subjected to PCR for detection of Mycoplasma sp. and MG. PCR products from all the samples showed a band size of 715bp which was the expected band size for Mycoplasma sp (Figure 2). Similarly, MG specific PCR showed a band size of 185bp showing that all the samples were positive for MG (Figure 3). Histopathology revealed that trachea had a thick layer of necrotic material in the tracheal lumen with the loss of epithelium and cilia and lymphocytic aggregation. Air sac showed necrotic material adhered with lymphocytic infiltration in the air sac wall while lung showed edema in the lumen, congestion of capillaries and lymphoplasmacytic infiltration in the interstitial tissue. Infra orbital sinus showed necrotic material in the lumen with lymphocytic infiltration (Figure 4).
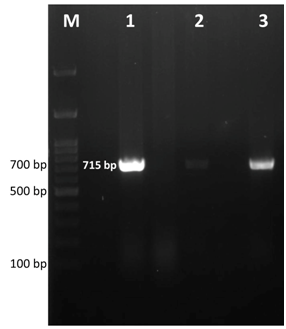
Figure 2 Gel electrophoresis image of Mycoplasma sp. specific PCR products. Lane M- 100bp ladder, Lane 1, 2, 3- positive samples showing expected band size at 715bp.
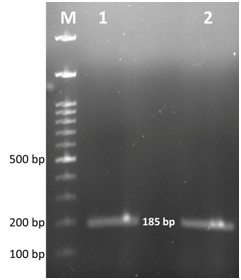
Figure 3 Gel electrophoresis image of Mycoplasma gallisepticum specific PCR products. Lane M- 100bp ladder, Lane 1, 2- positive samples showing expected band size at 185bp.
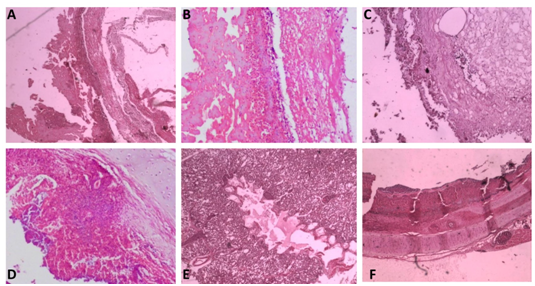
Figure 4 Histopathological observation.
(A) Air sac - 4 xs- Necrotic material adhered with the air sac wall
(B) Air sac - 4 xs- Necrotic material adhered with lymphocytic infiltration in the air sac wall
(C) Infraorbital sinus- 10x- Necrotic material in the lumen with lymphocytic infiltration
(D) Lung- 4x- Lung tissue infiltrated with lymphocytes and plasma cells
(E) Lung- 4x- Edema in the lumen, congestion of capillaries and lymphoplasmacytic infiltration in the interstitial tissue
(F) Trachea- 4x- Thick layer of necrotic material in the tracheal lumen with loss of epithelium and cilia and lymphocytic aggregation.
To the best of our knowledge, there are few reports on the molecular and pathological identification of MG in native chicken breeds of India. Thus there is an immediate need to screen native chickens against MG so as to sketch a better control strategy. Rapid diagnostic techniques like PCR can be employed to diagnose CRD as conventional methods are time consuming.
The authors are thankful to Tamil Nadu Veterinary and Animal Sciences University for supporting this work.
The authors declare that they have no competing interest.

©2018 Karthik, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.