Journal of
eISSN: 2377-4312


Research Article Volume 5 Issue 4
Department of Animal Pathology, University of Chile, Chile
Correspondence: Gustavo Farias R, Pathophysiology and Toxicology, Department of Animal Pathology Medicine, University of Chile, Santa Rosa 11.735, La Pintana Santiago, Chile, Tel 562 2978 5543
Received: December 27, 2016 | Published: June 6, 2017
Citation: Farias G, Frohlich E, Sanhueza P, et al. Differential diagnosis of scrapie in post mortem goats by immuno histo chemistry. J Dairy Vet Anim Res.2017;5(4):122-125. DOI: 10.15406/jdvar.2017.05.00147
Scrapie is a neurodegenerative disease of slow and lethal progression affecting sheep, goats and mouflons, which is part of a group of pathologies produced by prions or transmissible spongiform diseases (TSEs), these affect both humans and animals. Based on the fact that immunohistochemistry (IHC) is the internationally recognized method for the diagnosis of classical scrapie in goats, a species considered as a reservoir for the disease that may be zoonotic, we aimed to detect and differentiate it from other lesions or pathologies of the central nervous system. Chile in relation to bovine spongiform encephalopathy (BSE), is classified as a disease-free country. Scrapie is actively monitored for the sheep, but passively for the caprine species, which is why this study was carried out in slaughtered goats in the central north of the country (IV Region), to provide information to the surveillance system for this disease. Fifty brains of goats older than 2years were used, regardless of race or gender. The obex was taken from each of them, two serial histological sections were obtained, one used for the staining of Hematoxylin and Eosin (H/E) (50 cuts), which allowed us to observe lesions and to evaluate the aptitude of the samples. The other one was used for immuno histo chemistry (IHC) (50 cuts), which allows to locate prions in situ, using a commercial kit and the technique described by Manning et al.1 For a correct diagnosis, 4 controls were used, 2 positive (1 ovine and 1 caprine) and 2 negative (1 ovine and 1 caprine), provided by an International Reference Center. Analysis of the 50 H/E histological sections indicated that all were eligible for the IHC technique, and non-specific lesions (38/50) were found, such as focal haemorrhage, perivascular infiltrate and pigments, all of which are not attributable to prions. With the IHC technique, all the homologous histological sections of the samples and the negative controls were classified as negative to classic scrapie, due to the fact that they did not present immunostaining characterized by a red granular precipitate, which was present in the positive controls used. The results obtained in this work validate the application of the IHC technique at the level of obex, as a method of diagnosis of classic goat scrapie in the country.
Keywords: goats, tses, scrapie, prion, IHC
Transmissible Spongiform Encephalopathies (TSEs), or prion diseases, are a group of progressive neurodegenerative pathologies that affect the central nervous system (CNS) of a large number of mammals such as sheep and goats (Scrapie), bovine (Bovine Spongiform Encephalopathy or BSE), orhumans (Creuzfeldt-Jakob disease).2 The etiological agent of these diseases is an infectious proteincalled prion described by Prusiner3as a hydrophobic protein. Scrapie, the prototype of TSEs, was discovered more than 250years ago in Britain and other Eastern European countries, affecting sheep, goats and mouflons. In recent years, this pathology has become of great importance since there are evidences that relate the Scrapie prion to the bovine prion (BSE) and with a variant that affects humans, the reason why it is considered a zoonosis.4 The incidence described in goats is low compared to sheep, this may be a result of the lack of vigilance given to the species. The importance of this species began to emerge at the time that it was detected that a goat with the disease can propagate scrapie between its species and also to sheep.5
At the international level, immunohistochemistry (IHC), is the standard method of detection of scrapie in goats, this technique detects prions in the tissue of the CNS or in lymph nodes. In other species such as cattle or sheep, other diagnostic tests, such as the western blot or the ELISA test, these ones have not been widely used in goats.1,6 TSEs are closely related to the infectious variant of Creutzfeldt-Jakob disease in humans because it is a zoonosis caused by the consumption of animal products contaminated with BSE, for this reason the concern of nations with regard to TSEs, which has led to trade restrictions that have negatively impacted the bovine, ovine and caprine industries.1,4 Chile is classified as insignificant risk for BSE, however, there is no declared health status with respect to scrapie. Therefore, the main objective of this study was to contribute to the annual passive surveillance for this pathology, since the goats are a species considered reservoir for the prion. Thus, the more advanced the technology and more research on scrapie are, the better control of this disease, hence the importance of active annual surveillance, to expand awareness of the country's health status and contribute to epidemiological studies.
Animals
50 brains of goats older than 2years were used, regardless of race or gender, slaughtered in slaughter houses of North-Central Zone of Chile (Region IV).
Data Collection
Obex was taken from each brain, and fixed in 10% formalin for 7days, then processed and included in paraffin in an automatic equipment for 16hours. Serial sections of 5μm thickness were obtained, which were mounted on 2 slides: one for Hematoxylin-Eosin staining (H/E) to establish his ability and histopathological analysis; the other was used to perform the IHC technique (Pullman kit), developed according to internacional standards.7 For this purpose, positive and negative controls were used by the reference laboratory for Scrapie (National Reference Laboratory For Scrapie and CWD, Animal Research Institute, Canadian Food Inspection Agency).
Hematoxylin - Eosin (H/E)
This technique consists of two stains, one is Hematoxylin that has a deep blue-purple color and stains nucleic acids and the other consist in the Eosin that is pink and stains proteins non specifically. With this technique, it was possible to verify the aptitude of the sample, evaluated from the observation of the integrity of different nerve nuclei of bilateral presentation.8,9 In addition, the histopathological study was performed to describe different lesions suggestive of other pathologies.9
Immuno histo chemistry (IHC)
Samples were subjected to the established protocol including inhibition of the endogenous activity of the peroxidase enzyme present in the tissue, through incubation in 3% hydrogen peroxide in absolute methanol for 10min. Then, the samples were immersed in double-distilled water and incubated in 98% formic acid for 5min, washed and neutralized in a 0.1M Tris-HCl buffer solution, pH 7.6 for 2min. They were transferred to an antigen retrieval solution pH 7.6 (Target Retrieval Solution®) and held in an autoclave for 20min at 120°C and then incubated in a Tris Saline-Tween 20 Buffer solution (TBST) pH 7.6 per 10min.1,9 The protocol of the Pullman kit (Monoclonal F99/97.6.1) was followed for immuno staining. Samples were incubated in a proteinase K solution for 90sec at room temperature, followed by 3 washes with TBST pH 7.6. Subsequently, incubated with the F99/97.6.1 monoclonal primary antibody in a 1:1000 dilution, for 20min at 37°C, followed by 3 washes with TBST pH 7.6. They were incubated with the biotinylated anti-mouse polyclonal secondary antibody for 15min at 37°C, followed by Streptavidin-horse radish peroxidase (HRP) for another 10min. Finishing with the development of the immunological reaction, by the protected incubation from direct light with 3-amino-9-ethylcarbazole (AEC) for 5min. Finally the parenchyma of the adjacent tissue was stained with a contrast solution (Mayer's Hematoxylin) for 10min. Each sample was compared to the controls, positive and negative, provided by the reference laboratory. Determining a sample as positive in the presence of specific immunostaining in the tissue accompanied by symmetric and bilateral lesions; and negative in complete absence of immunostaining and vacuolar lesions.1,9
H/E
The 50 goat obex samples analyzed were able to perform the IHC. The main alterations observed in the tissues were: focal haemorrhage (30) (Figure 1), perivascular infiltrate and pigments. No lesions similar to those produced by prions were found. Although if other types of injuries were found in 38/50 cuts, it is concluded that these were not associated with classic scrapie, if not others causes or pathologies (Table 1).
IHC
Obtained results indicate that the 2/2 positive controls (goat and sheep) (Figure 2), always presented the red granular immunoprecipitate, in addition to vacuoles that indicate the presence of the prion associated with scrapie, and that agrees with the described by other authors.1,10 Unlike the 2/2 negative controls and all 50/50 samples of national goats obex (Figure 3), in which immuno precipítate was not observed, nor were vacuoles or spongioses of nervous tissue. This confirms that 100% of the analyzed samples are negative to classic scrapie (Table 2).
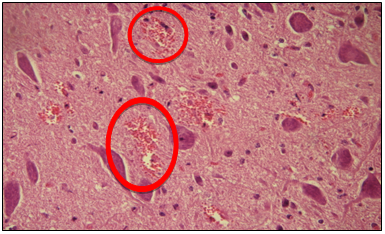
Figure 1 Microphotography of a sample, at the level of the obex in which the presence of focal hemorrhages (circles) is observed (H/E 400x). The structure of the nervous tissue is normal, with neuronal sums, and no vacuoles or spongiosis are observed.
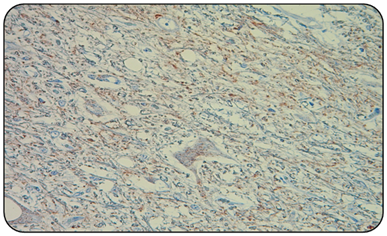
Figure 2 Microphotography of a goat obex positive control (IHC 400x). The presence of numerous vacuoles or spongioses of the nervous tissue, at the level of pericarion and of the neuropilo, and the immunostaining of red granular precipitate.
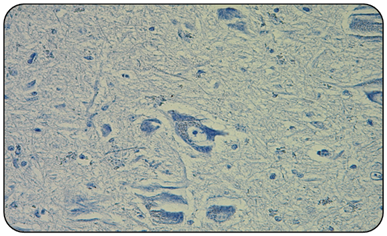
Figure 3 Microphotography of a goat obex negative control (IHC 400x). In which normal morphology of the nervous tissue and neuronal sums is observed. No vacuoles or the presence of immunostaining of red granular precipitate are observed.
Histopathological |
Neuropylus and |
Perivascular |
Focal |
Pigments |
No |
Total samples |
Number of samples |
0 |
6 |
30 |
2 |
12 |
50 |
Percentage of |
0% |
12% |
60% |
4% |
24% |
100% |
Table 1 Histopathological analysis of samples (H/E)
IHQ |
Number of samples |
Number of |
Total Samples |
Percentage of |
Negative |
50 |
2 |
52 |
96,3% |
Positive |
0 |
2 |
2 |
3,7% |
Suspects |
0 |
0 |
0 |
0% |
Total Samples Analyzed |
50 |
4 |
54 |
100% |
Table 2 Immunohistochemical analysis of samples (IHQ)
Scrapie in goats, like all diseases caused by prions, is neurodegenerative and fatal, slow progression with no inflammatory or immune response, which reduces the chances of early detection in affected animals.11 The importance of TSEs lies in the zoonotic characteristic of strains that affect cattle (BSE), this represents a problem both economically and for public health. According to the OIE, the scrapie is the eighth causes of sheep and goat loss in the world .12 Undoubtedly the greatest risk is the passage of the prion of BSE into the human food chain, and, although there is no evidence that the goat prion is able to cause some effect in humans, the alert regarding the prions that affect the small ruminants are the existence of different strains to the classic, whose behavior remains unpredictable and their characterizations are still not well understood.13 Thus, the occurrence of BSE strains detected in one goat in France in 2002 and a second case in the United Kingdom, raised the first questions regarding the existence of strains other than the classic one, which must be detected and analyzed for their subsequent risk assessment.14 What has been demonstrated is the transmission of BSE to sheep, thus making scrapie a potential threat to human health and to goats and sheep as a prion reservoir.15 This initial work, aim to apply, standardize and validate in the country conditions, the IHC technique used to detect classic scrapie in chile an goats and thus to encourage their active surveillance.
The true scrapie situation in many countries of South America is still unknown, because there is usually an inadequate surveillance system that is generally passive to detect affected animals. Thus it is almost impossible to establish the real state of freedom of the disease, without first establishing a standardized diagnostic system that allows an active surveillance. In this context, the ability of early and safe detection is vital for scrapie diagnosis to have real value. Particularly in animals destined for human consumption, where there is an additional requirement to determine the presence of the prion, hence our choice to use CNS from apparently healthy and clinically asymptomatic slaughter house goats that are destined for consumption.6,16
Despite the longer time required for its performance, compared to the so-called "rapid" tests such as ELISA and Western Blot,17 the IHC has important advantages, since it can be performed in tissues other than CNS tissue achieving excellent results.10 It is possible to affirm that immunohistochemistry is the most reliable, secure and confirmatory test for the diagnosis of scrapie, in relation to other immunodiagnostic techniques, such as rapid tests, and it is recognized by the OIE since 2000.
In this work, of a total of 50 animals, of which 100 serial histological sections of obex were used, all of them presented the established ability to be submitted to IHC technique. Coincidentally for this study, only animals over 2 years of age were used, animals that arrived at a slaughter house without clinical suspicion. In addition, when analyzing H/E histopathology samples, it was possible to detect the nuclei of the solitary tract, the spinal tract of the trigeminal nerve and the dorsal or parasympathetic nerve of the vagus nerve, necessary for diagnosis.18 On the other hand, in these sections it was possible to observe lesions of different types in 38 of the 50 analyzed samples, however none of these were associated with scrapie but other causes.
The IHC test performed in this study was based on a commercial kit containing the monoclonal antibody specific for detecting the PrPSc and chromogen AEC to reveal the reaction and which has already been used by other authors in nervous tissue and othertissues, from different productive species of Chile.17 The utility and correct application of the kit were validated by obtaining the expected immunoreaction in the positive controls provided by the reference center. Thus, all the histological sections obtained from obex of goats from the country were negative to scrapie, which coincided with other studies obtained in the ovine specie.17
However, it should be added that this study involved a random selection of animals, with a reduced sample section (50 individuals), therefore the available information regarding the sanitary condition in the caprine species remains uncertain. It should be noted that most of the country's goat mass is in the hands of the most humble socioeconomic sector, small producers, who mainly market their meat and Dairy products in the informal market.19 On the other hand, when analyzing the results of the immunoreaction that were presented with the IHC, they coincided with those found in bovine samples. In conclusion, as in all of the samples, no specific immunostaining was observed, as well as nor vacuolar lesions in the specific anatomical areas of the obex, all samples were negative to scrapie with the immuno histochemical technique. In this case, the immunohistochemical method was performed under the conditions of the official diagnostic laboratory of TSEs in Chile, which presented 100% sensitivity and specificity for scrapie, which agrees with Manning et al.1 In the case of the samples used as controls for the IHC, provided by the reference center, 2 sheep and 2 goats, showed that in all the cuts of obex, all the positive ones always presented the red granular immuno precipitate, indicative of the immuno reaction of the antibody with PrPSc, indicating its location in the affected nerve nuclei and visualization of vacuoles in the nucleus of the solitary tract, which were never observed in the negative controls
None.
Author declares that there is no conflict of interest.

©2017 Farias, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.