Journal of
eISSN: 2377-4312


Mini Review Volume 5 Issue 1
1Department of Stem Cells and Developmental Biology, Royan Institute for Stem Cell Biology and Technology, Iran
2Department of Physiology, University of Medical Sciences, Iran
3North Carolina State University, USA
Correspondence: Maryam Farzaneh, Department of Stem Cells and Developmental Biology, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, Tehran, Iran, Tel 989 104 579 736, Fax 98 19395 4644
Received: November 15, 2016 | Published: January 10, 2017
Citation: Farzaneh M, Khoshnam SE, Mozdziak PE. Concise review: avian multipotent stem cells as a novel tool for investigating cell-based therapies. J Dairy Vet Anim Res. 2017;5(1):1-4. DOI: 10.15406/jdvar.2017.05.00125
The chick embryo is a favorite and great in vivo model, which has long been used in embryology, developmental biology and applied sciences. Also, chicken embryo is the accessible model for transplantation and tracking studies. Avian multipotent stem cells have the capability to self-renew and differentiate into a variety of cell types in vitro. Avian multipotent stem cells were successfully isolated from bone marrow (BM), metanephric tissue, lung, amniotic cells, cartilage, and other embryonic and adult tissues. Subsequent analysis was performed to evaluate these multipotent cells in vitro. Results of the present study strongly contribute to the information related to the ability of avian multipotent stem cells to differentiate into cells such as adipocytes, osteoblasts, chondrocytes, astrocytes, and other committed cells. Avian multipotent stem cells may provide a new source of avian-derived cell lines for basic research and potential therapeutic applications.
Keywords: avian stem cells, multipotent stem cells, mesenchymal stem cells, regenerative medicine
One of the first most important events in the avian embryonic development is the specification of the three embryonic lineage including ectoderm, mesoderm, and endoderm.1,2 The commitment of pluripotent embryonic cells into these groups requires the continue action of several gene products. Although, in the adult tissue, the presence of multipotent to unipotent cells have been considered to maintain some tissue regeneration.3‒7 Avian embryos are a great model in basic and applied sciences.8,9 Avian multipotent stem cells can maintain self-renewal and multilineage differentiation ability in vitro.10 The vast majority of avian multipotent stem cells have been obtained from different tissue sources including bone marrow, primary kidney, lung, dermis, and other tissues and the stem cell populations derived from these tissues are valuable (Figure 1). Among different kinds of multipotent stem cells, mesenchymal stem cells are the most representative type, which can be differentiate into muscle, adipocytes, cartilage, and osteoblasts in vitro.11 However, the fate of multipotent stem cells was believed to differentiate only into the tissue or organ from which they originated.6,12 Although, previous reports have suggested that in specific environmental conditions, the multipotent cells may undergo unexpected fate.6,12 The appropriate effects of multipotent stem cells are in cell-based therapy, tissue engineering, cell transplantation, transgenic studies, and viral vaccines production.9,13‒16 Therefore, these cells may provide a new source of avian-derived cell lines for basic research and potential therapeutic applications.17‒19
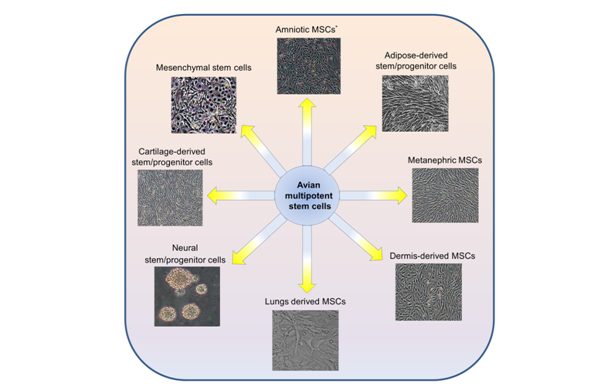
Avian mesenchymal stem cells
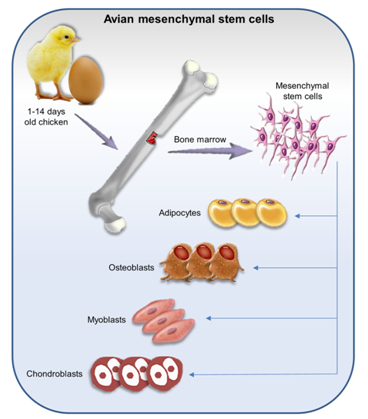
Avian mesenchymal stem cells (MSCs) are a small population and commonly isolated from adult bone marrow (BM).10,12,20,21 MSCs are fibroblast-like multipotent progenitor cells which have the ability for self-renewal and a differentiation capacity.22 MSCs were isolated from different anatomical regions of chicken embryo23 and postnatal 1-14days old chicken.11,20 MSCs have been isolated from lung,22 adipose tissue, and skeletal muscle, and metanephric tissue, as well.14 These cells are positive for classic embryonic stem cells that are associated with self-renewal (Table 1)20,24 and differentiation capacity into muscle, adipocytes, cartilage, and osteoblasts in vitro (Figure 2).6,24,25 Moreover, they have immunoregulatory function and inhibit immunological response,26,27 therefore, these cells are permissive to infected with a variety of pathogenic viruses such as infectious bursal disease virus (IBDV)13 which does not proliferate in chicken fibroblast cells.24 MSCs are a great tool for a wide variety of diseases research such as cell transplantation, regenerative medicine, and production of transgenic chickens.12,28
Avian metanephric mesenchymal stem cells
Avian metanephric mesenchymal stem cells (MMSCs) was obtained from 20days old Beijing duck metanephros.14 The expression of related genes and proteins, self-renewal potential, their differentiation, and similar morphology with MSCs were evaluated.14,29 However, their specific surface markers are unknown and they identified based on the expression of certain markers associated with MSCs (Table 1). It seems that, MMSCs are pluripotent and able to differentiate into three somatic lineages such as epithelial cells, liver cells, and pancreatic cells.14 There are few reports of harvesting avian MMSCs; while, MMSCs are applicable for tissue engineering, cell transplantation, and cell therapy applications on the kidney.14,30
Cell Type |
Surface Marker |
Multipotent Transcription Factor |
Differentiation Potential |
Mesenchymal Stem cells (MSCs) |
CD44, CD44, CD90, CD105 |
Oct4, Nanog, Sox2 |
Adipocytes, Osteoblasts, Chondroblasts, Myoblasts |
Metanephric MSCs (MMSCs) |
CD73, CD71, CD44, CD34, CD29, CD45, Pax2, CD166 |
Not determined |
Adipocytes, Hepatocytes, Epithelial cells, Islet cells |
Lungs Derived MSCs (LMSCs) |
CD44, CD90, CD105 |
4-Oct |
Adipocytes, Osteocytes |
Amniotic MSCs (AMSCs) |
CK19, CD29, CD44 |
Oct4, Nanog, Sox2 |
Adipocytes, Osteoblasts, Myocardial cells |
Cartilage-derived stem/Progenitor cells (CSPCs) |
CD29, CD44, CD166, CD105 |
Not determined |
Adipocytes, Osteoblasts, Chondrocytes |
Neural stem/Progenitor cells (NSPCs) |
Not determined |
Not determined |
Neurons, Astrocytes, Oligodendrocytes |
Dermis-derived Mesenchymal stem/progenitor cells (DMS/PCs) |
CD44, CD71, CD73, β-integrin |
Not determined |
Osteoblasts, Adipocytes, Neurocytes |
Adipose-derived stem/Progenitor cells (ADPCs) |
CD29, CD44, CD71, CD73 |
Not determined |
Osteoblasts, Adipocytes, Myocardial cells |
Table 1 Characterization of avian multipotent stem cells
Avian lung mesenchymal stem cells
Avian Lungs derived mesenchymal stem cells (LMSCs) were obtained from 1 to 2 weeks old chicken. Chicken LMSCs similar to human31 and mouse LMSCs32 are multipotent cells with a fibroblast-like morphology, expression of self-renewal and pluripotency marker (Table 1) and differentiation into adipocytes and osteocytes.22 LMSCs have immuneregulatory properties by the inhibition of T-cell proliferation; indeed they have immunosuppressive effects. LMSCs are susceptible to infection with pathogenic viruses and suitable target for viral replication and apoptosis. LMSCs are valuable source for cell therapy applications on the lung and as target for virus infection.22
Avian amniotic mesenchymal stem cells
Avian amniotic mesenchymal stem cells (AMSCs) were obtained from amniotic epithelial cells.33 AMSCs are multipotent cells and express transcription factors which are essential for maintaining the self-renewal and undifferentiated state (Table 1). AMSCs are able to give rise into osteoblasts, adipocytes, myocardial cells,34 neural-like cells, and islet like cells.33 AMSCs suitable tool for tissue engineering research and autologous cell therapy.34‒36
Avian cartilage stem/progenitor cells
Avian cartilage-derived stem/progenitor cells (CSPCs) were obtained from the surface zone of articular cartilage from 20days old chicken embryo. CSPCs are uniform population with long fusiform or polygon morphology.37 CSPCs are multipotent stem cells and identify by expression of specific surface markers, ability to self-renew, and differentiate into three cell types such as adipocytes, osteoblasts, and chondrocytes.37,38 These cells are great tools for cell-based cartilage repair.37,39-41
Avian neural stem/progenitor cells
Avian neural stem and progenitor cells (NSPCs) were isolated from dorsal ventricular ridges of 10-13days old chick embryos.42 NSPCs are round neurospheres which able to self-renew and differentiate into neurons, astrocytes, and oligodendrocytes.42 These cells are suitable for gene therapy, intracellular distribution of proteins, neurogenesis, tumorigenesis, and given rise to many interesting findings.42‒45
Avian dermis-derived mesenchymal stem/progenitor cells
Avian dermis-derived mesenchymal stem/progenitor cells (DMS/PCs) were obtained from dermis of 16days old chick embryos.46 These cells show fibroblast-like morphology and multilineage differentiation into adipocyte, osteocytes, and neural cells.46 DMS/PCs exhibit extensive applications in stem-cell based therapy and tissue regeneration study.46‒48
Avian adipose-derived stem/progenitor cells
Avian adipose-derived stem/progenitor cells (ADPCs) were isolated from subcutaneous tissues of the abdomen and inguinal fat pads of 1 day old chickens.49 ADPCs are fibroblast-like multipotent stem cells with self-renewal ability and differentiation into osteoblasts, adipocytes, and myocardial cells in vitro.17,49 These cells are novel tool for regenerative medicine, as well.17,49‒53
The biochemical and molecular markers for detecting and evaluating avian multipotent stem cells have yet to be developed (Table 1). In vitro studies have also demonstrated the advantages of using multipotent stem cells for cell transplantation. Actually, very few researches exist that incorporate the beneficial effects of avian multipotent stem cells. Collectively, these data demonstrate that research on the properties of avian multipotent stem cells have led to renewed interest in their extended applications.
This study was financially supported by grants provided from Royan Institute and Iranian Council of Stem Cell Technology and the Iran National Science Foundation (INSF).
Author declares that there is no conflict of interest.

©2017 Farzaneh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.