Journal of
eISSN: 2377-4312


Research Article Volume 3 Issue 3
1Federal College of Animal Health & Production Technology, Nigeria
2Department of Production Animal Studies, University of Pretoria, South Africa
Correspondence: Olubunmi Gabriel Fasanmi, Federal College of Animal Health & Production Technology, Moor Plantation, Ibadan, Nigeria, Tel 27625047537
Received: May 04, 2016 | Published: May 30, 2016
Citation: Okuneye OJ, Ogunfolabo LA, Fasanmi OG, et al. Performance and physiological responses of Salmonella Enteritidis challenged broilers fed diets containing antibiotic, probiotic and aromabiotic. J Dairy Vet Anim Res. 2016;3(3):106-110. DOI: 10.15406/jdvar.2016.03.00081
The problems of salmonella zoonosis and antibiotic resistance leading to drop in productivity and performance in poultry and the negative effects on public health is persistent especially in third world countries. This study was designed to evaluate the performance and physiological responses of Salmonella enteritidis challenged broilers fed diets containing antibiotic, probiotic and aromabiotic. One hundred and twenty 8 weeks old commercial broilers were allotted into four treatment groups, T1 (control), T2 (antibiotic), T3 (Probiotic) and T4 (Aromabiotic) each replicated thrice, with 10 birds per replicate. The feeding trial lasted for four (4) weeks, after which cultured Salmonella enteritidis was inoculated at the dose rate of 107cfu orally. Birds were weighed on weekly basis, feed consumed and other performance parameters calculated, and blood samples were collected for both haematological and serum biochemical analysis 1, 3, 5, 7 and 9day post infection (dpi). Data generated were subjected to analysis of variance (ANOVA) and means separated using Duncan multiple range test for significance (P<0.05). The results showed that T3 with probiotic has highest mean values for weight gain, lowest mortality and FCR better at P<0.05 than other treatment groups, also there were significant (P<0.05) differences on PCV, [Hb], WBC, Lymphocyte and Platelets across the treatment groups, T3 and T4 had significantly (P<0.05) higher values than T1 and T2 in these haematological parameters. T3 with probiotics has lowest mean values for ALT, AST, BUN, creatinine, triglycerides and cholesterol, no mortality was recorded before and after experimental infection in T3, while other treatment groups recorded varying degrees of mortality. Irrespective of the dpi, this trend was maintained, but as the severity of the infection progresses the values of all the parameters dropped until 9dpi, the mean values of globulin was maintained in the probiotic group (T3) throughout the post- infection period. Therefore it can be concluded that Probiotic supplementation improved performance, increased the immunity of the birds to salmonella challenge and does not have adverse effect on kidney and liver functions. Probiotic (Protexin®) can be administered at a dose rate of 7.5g/kg of broiler feed.
Keywords: antibiotic, aromabiotic, performance, physiology, probiotic, serum, Salmonella enteritidis
Epidemiological reports suggest that poultry meat is still the primary cause of human salmonella food poisoning,1 and the prime suspect more often than not is Salmonella enteritidis,2,3 this can arise through contamination of fresh and processed meat from the microflora of poultry, slaughtering premises, operators’ hands, equipment and outfit, water and air.4‒6 The presence of salmonella in poultry flocks and the emergence of salmonellosis has caused great economic losses in poultry production.7 It is often difficult to ascertain how Salmonella issues in poultry begin and what measures should be implemented to prevent them. Chickens may become infected through both vertical and horizontal means.8 Salmonella enteritidis is known to be invasive in chicks, resulting in high mortality in broiler flocks.9 Concerns about food safety have prompted the poultry industry and decision makers to introduce control plans to combat Salmonella.10
For many years, various antimicrobials were used in feed to treat and prevent infections in poultry. However, a lot of bacteria (including Salmonella) become resistant to one and in many cases to multiple antimicrobials. This resistance is proving fatal for thousands of people every year and results in high medical and economic costs. In addition, from the consumers’ point of view meat should be free from both pathogens and antimicrobial residues.11 Recent restrictions on the use of some antimicrobials as growth promoters in animal production have pressured the poultry industry to look for alternatives that can continue to provide performance benefits. Aromabiotic, Prebiotic, Synbiotic, Probiotic etc., have been evaluated for this purpose with some success.12,13 Probiotics and aromabiotics have demonstrated significant potential as therapeutic options with antimicrobial activity for a variety of diseases and salmonella infections,14 but the mechanisms responsible for these effects have not been fully elucidated.
This study seeks to evaluate the effects of growth promoters on broilers challenged with oral dose of Salmonella enteritidis and also study the comparative effects of aromabiotic, probiotic and antibiotic on haematology and serum biochemistry of broilers challenged with oral dose of Salmonella enteritidis.
Experimental sites and materials
The experiment was carried out at the Student Teaching and Research Farm, Poultry Section of the Federal College of Animal Health and Production Technology, Moor Plantation, Bora, Apata. Ibadan. One hundred and thirty (130)day old Arbor acre chicks were purchased from a reputable hatchery in Ibadan. The chick's were acclimatized for a period of four weeks (4) after which one hundred and twenty (120) birds were randomly selected, weighed and allotted into four dietary treatments, T1, T2, T3 and T4 respectively, with each treatment replicated thrice. Ten birds were allotted into each replicate. Different growth promoters were included in the diet of the respective treatments in line with the manufacturer’s recommendation.
Birds were fed Ad-libitum on treatment basis, feeds were weighed before they are served and the left-over. Birds were weighed on weekly basis; the birds were fed on these diets for Four weeks (4wks). Thereafter, cultured Salmonella enteritidis obtained from the Laboratory of Animal Care Services, Ogere, Ogun State. Serial dilution was done to 107cfu and administered orally through drinking water. Twenty-four hours (24hrs) after the administration of the cultured Salmonella enteritidis, blood samples were collected for hematological and serum biochemical analysis. Two birds were selected and tagged from each replicate for the collection of 3ml of blood sample which was done through the wing vein of the birds with 19 gauge needle and 5ml syringe, two sets of sterilized labelled sample bottles, one containing Ethylene Diamine Tetraacetic Acid (EDTA) and other bottles without anti-coagulant. Blood was collected, kept on ice in a cooler and transferred to the laboratory for analysis. Subsequent blood collections were done every 48hrs for four (4) consecutive times.
Laboratory analysis
Hematological and serum biochemical analyses were carried out at the laboratory of Department of Veterinary Physiology, University of Ibadan. Blood collected were analysed for hematological parameters (PCV, [Hb], RBC, WBC, Platelets, MCV, MCH, LYM, MCHC) and serum biochemical (Albumin, Globulin, Cholesterol, Triglyceride, Creatinine, ALT and Blood Urea Nitrogen) These samples were immediately used for determination of haematological parameters, Haemoglobin concentration was determined spectrophotometrically, as described by Franceschini et al.,15 Packed cell volume (PCV) and Red Blood Cell (RBC) counts were determined as described by Dacie & Lewis.16 Total White Blood Cell (WBC) counts were determined using Neubauer haemocytometer. Blood constants (Mean Corpuscular Volume, Mean Corpuscular Haemoglobin and Mean Corpuscular Haemoglobin Concentration) were determined using appropriate formulae as described by Jain, in Veterinary haematology.17 The second sets of samples without EDTA were centrifuged and serum decanted for serum biochemical analysis. Total serum protein was determined using appropriate kits with basic procedure of Kohn & Allen,18 albumin was measured using bromocresol green (BCG) binding technique as described by Abdul Hameed,19 while serum cholesterol was determined by the Roschlan methods. The proximate composition of the broiler starter and finisher mash was determined according to the official method of analysis.20 Data collected were subjected to analysis of variance using,21 and where significant differences occur, the means were separated by Duncan multiple range test.
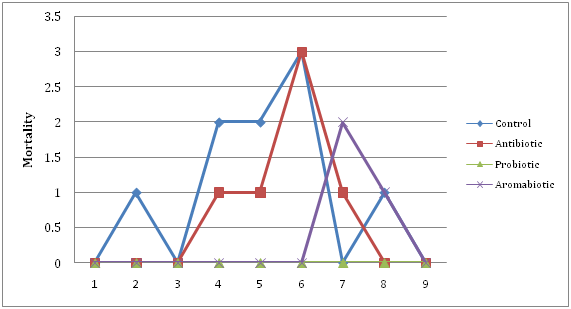
Commercial poultry is one of the fastest growing sectors of the animal agricultural industry, especially broiler production,22 an increase in consumption of meat and poultry increases the potential risk for exposure to Salmonella through contamination. As pressure to reduce and ultimately eliminate the use of antibiotics in poultry feed has increased, food safety standards have also been heightened. This research work proved beyond reasonable doubt that whenever commercial broilers are fed diets containing feed-grade organic growth promoters (probiotic or aromabiotic) and there is infection of the bird, as it was experimentally demonstrated, there is bound to be some level of protection against bacteria pathogens especially Salmonella enteritidis due to their antibacterial properties, also there were no adverse effects on the haematological and serum biochemical parameters of the birds 1, 3, 5, 7 and 9day post infection (dpi). Following the experimental infection, observations thereafter for the mortality recorded was highest for the T1 (control) , followed by T2 (antibiotics) and T4 (aromabiotic), while T3, broilers fed diet containing probiotic recorded no mortality throughout (Figure 1), this in line with the findings of23 that low-grade damage to the intestinal tract by pathogenic bacteria may cause poor feed conversion efficiency and decreased rate of body weight gain in poultry flocks, but more severe enteric damages by salmonella infections will result in overt disease and high mortality.
Feed is probably the most important entity in the poultry industry that can expose the birds to a wide variety of factors through the gastrointestinal tract.24 The gastrointestinal tract microflora is a mixture of bacteria, fungi, and protozoa, but bacteria are the predominant microorganisms.25,26 Because different bacterial species have different substrate preferences and growth requirements, the chemical composition of the digesta, to a large extent, determines the compositions of the microbial community in the gastrointestinal tract.27 In view of this the broiler starter and finisher diets were formulated in order to meet the nutritional needs of commercial broilers and gut conditions (Appendix 1). Following the addition of the various growth promoters into the diets fed to the birds in the respective treatments T1 (control), T2 (antibiotics), T3 (probiotics) and T4 (aromabiotics) over a period of 4wks, the performance characteristics for each of them is as stated below (Table 1).
Variables |
T1 |
T2 |
T3 |
T4 |
±SEM |
Control |
Antibiotics |
Probiotics |
Aromabiotics |
||
Initial weight(kg) |
0.76 |
0.76 |
0.77 |
0.76 |
0.01 |
Final weight(kg) |
1.30c |
1.35bc |
1.50a |
1.40b |
0.15 |
Av. Weight gain(kg/4wk) |
0.54c |
0.59bc |
0.73a |
0.64b |
0.2 |
Av. Weight gain(kg/wk) |
0.14c |
0.15bc |
0.18a |
0.16b |
0.02 |
Av. Feed intake(kg/4wk) |
1.45b |
1.46b |
1.56a |
1.51a |
0.06 |
Av. Feed intake(kg/wk) |
0.36 |
0.37 |
0.38 |
0.38 |
0.01 |
Feed Conversion Ratio |
2.69a |
2.47b |
2.14d |
2.36c |
0.26 |
*Mortality(%) |
16.67 |
16.67 |
0 |
0 |
|
Table 1 Performance characteristics of broilers fed diets containing antibiotics, probiotics and aromabiotics
abcd, mean values along the same row with different superscripts are significantly different(P<0.05)
*Mortality recorded before challenge with Salmonella enteritidis
Significant differences at P<0.05 were observed in four of the parameters (final weight, average weight gain, feed intake and feed conversion ratio) and % mortality (before the birds were challenged with Salmonella enteritidis) measured and compared across the treatments. T3 (with probiotic) is greater than all other treatments in the final weight, average weight gain and feed intake; this is followed by T4 (with aromabiotic), while there were no significant differences in all the three parameters measured between T1 and T2 (antibiotics). The birds in T3 had the best performance in average weight gain because probiotics are made up of lactobacillus predominantly, which are favourably disposed to good gut health, thereby facilitating the growth of beneficial group of gut microbes and depressing the potentially pathogenic and harmful group,27 this will in turn favour digestion of food and assimilation of the end products thereof, which will be used for muscle or flesh formation needed for weight gain , which is seen as lowest value for the feed conversion ratio (FCR). On the other hand, the addition of antibiotics (T2) did not produce significant change to the parameters measured when compared with T4, because the antibiotic does not produce significant positive effect on the gut microbes, this is in agreement with the submission of,7 that the most frequently isolated strain in poultry flocks, S. Enteritidis, has shown considerable resistance to some antibiotics, this is as a result of the long-term use or abuse of certain antibiotics in poultry. All these effects occur at the expense of animal production performance,28‒30 hence the high FCR and low average weight gain. T4, which is the aromabiotic group, performed better than T1 and T2, this is because aromabiotic also has antimicrobial properties, which necessitated the better performance when compared with either or both T1 and T2, this is in agreement with31‒33 that non-inclusion of aromatic extracts or MCFA in the diet of commercial broilers led to decrease nutrient digestion and absorption, reduction in weight gain, and increases feed conversion ratio (FCR). There is a significant difference in % mortality recorded across the treatment groups, 16.66% was recorded for the control and the treatment group with antibiotics, while T3 had 0% and T4 with aromabiotic also recorded 0% mortality which is in consonance with the findings of.34,35 There is variation in the % mortality recorded between the antibiotics group and the organic growth promoters possibly because of the development of resistance of the gut microbes to antibiotics.7,36
Haematological and serum biochemical parameters are used as baseline in determination of damage to blood cells, essential for the diagnosis of various pathological and metabolic disorders due to impairment of some organs, they also provide valuable information on the immune status of animals.37 Haematological changes are commonly used to determine the health status and to assess the impact of environmental, nutritional and/or pathological stresses.38 Following the experimental infection of the birds in the different treatment groups, the collection and laboratory analysis of blood samples were done 1dpi, 3dpi, 5dpi, 7dpi and 9dpi, thereby giving rise to Tables 1‒5 respectively for haematological and serum biochemical parameters.
Table 2 shows the Haematological and serum biochemical parameters of Salmonella enteritidis challenged broilers fed diets containing antibiotic, probiotic and aromabiotic. T2 with antibiotics has a significantly lower PCV, [Hb], RBC, lymphocyte and platelets when compared with T1, T3 and T4, The high level of PCV for T1 and others may possibly be due to dehydration resulting from diarrhoea due to infection from Salmonella enteritidis,39 also, Chineke et al.40 documented that high PCV haematocrit reading indicated either an increase in the number of circulating RBC or reduction in circulating plasma volume. Birds fed probiotic showed the highest value for lymphocyte count, this may be as a result of the stimulation of the immune system by the immunogenic property of the probiotic used in this treatment,41 neutrophils especially are the first type of defence cells that will appear during acute infection causing due to the Salmonella enteritidis challenge, they are part of the immune system for protection of the birds,42 that is why the value is high in T1, T2 and T, with T3 having the least value because the birds in this treatment group have high level of immunity to microbial invasion.41 All other haematological parameters measured do not follow a particular order of significance or are statistically the same, but the value of all the haematological parameters fall within the normal range of values as determined by Elagib HAA.43
The treatment groups T3 and T4 with organic growth promoters have significantly greater (P<0.05) total protein and globulin levels more than T1 and T2, the high level of globulin which is a precursor for immunoglobulin (antibodies) is responsible for the protective functions of probiotic and aromabiotics.44 The immune system of birds is complex and is composed of several cells and soluble factors (proteins) that must work together to produce a protective immune response. A properly functioning immune system is of special importance to poultry because most commercial flocks are raised under intensive rearing conditions. Under such conditions, the flocks are vulnerable to rapid spread of infectious agents and disease outbreaks.45 The low levels of total protein and especially globulin in T1 and T2 when compared with T3 and T4 groups will cause hypoproteinemia generally and specifically hypoglobulinemia.46 This will lead to a fall in level of immunoglobulin (antibodies) leading to declining immune response47 and birds will lead to incidence of mortality,47,48 as seen in Figure 1.
Creatinine and blood urea nitrogen are indicators of the state of health of the kidneys, T3 (probiotic) has the least mean values for both parameters when compared with other treatment groups (T1, T2 and T4) as shown in Table 2, this implies that probiotic does not have adverse effect on the functionality of the kidneys in the commercial broilers in T3. ALT and AST which are referred to as liver serum enzymes, which ordinarily are not found in the blood, unless there is damage to liver cells, they are used to determine the state of health of the liver.49 The use of antibiotics (T2) and aromabiotics (T4) show a significant increase (P<0.5) in the AST and ALT levels when compared with probiotics group (T3), this is because infection due to Salmonella enteritidis can cause this,50 and possibly because of the development of resistance. The mean cholesterol and triglycerides levels for T3 is the least when compared with T1, T2 and T4, this is also a positive and endearing quality of probiotics, it will help the birds health-wise during summer or hot weather35 and will encourage good patronage by humans due to the risk attached to consumption of high level of cholesterol. All parameters that differ from each of the treatments still fall within the normal range of values as determined by Albokhadaim et al.51 All the parameters measured subsequently, 3dpi, 5dpi, 7pi and 9dpi are presented in Tables 3, 4, 5 & 6 respectively. Parameters in Tables 3, 4,& 6dropped in mean values possibly because of the severity of the challenge posed by Salmonella enteritidis as the infection progresses (Figure 1), but these parameters also followed the same trend as the presentation in Table 1 for 1 dpi. It was observed that all parameters dropped further 5 dpi except the lymphocyte counts that increased across board (Table 4), while in Table 5 (7 dpi) the serum biochemical parameters for all the treatments except aromabiotic treatment group increased, possibly because birds are gradually recovering and so there is a corresponding physiological response (Tables 2‒6). All the mean values for haematological and serum biochemical parameters in Table 6 (9 dpi) increased in all the treatment groups when compared with other Tables 2-5 due to the advance state of recovery from salmonellosis (Figure 1). There is a striking observation in Table 6 when compared with other Tables 2‒5, the globulin levels were maintained by the birds in the probiotic group (T3) throughout the post-infection period, but the globulin level dropped in all the other treatment groups (Table 6), this is an evidence responsible for probiotics to be able to perform the immuno-modulatory functions in poultry.52
This research work has proven beyond reasonable doubt that antibiotic usage in broiler feed has been discovered to be non-productive and dangerous to animal and possibly human health, while aromabiotic in broiler diet without Salmonella challenge will produce a very good performance in the FCR and liveability, but following field challenge with this bacteria the haematological and serum biochemical parameters will be affected negatively. Probiotic supplementation improved performance, increased the immunity of the birds to salmonella challenge and does not have adverse effects on kidney and liver functions. It is recommended that Probiotic (Protexin®) can be administered at a dose rate of 7.5g/kg of broiler feed.
None.
Author declares that there is no conflict of interest.

©2016 Okuneye, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.