Journal of
eISSN: 2374-6947


Research Article Volume 3 Issue 5
Department of Internal Medicine/Endocrinology, University of New Mexico, USA
Correspondence: Mark R Burge, Department of Internal Medicine/Endocrinology, University of New Mexico Health Sciences Center, Albuquerque, NM, USA, Tel 505 272 4658, Fax 505 272 5155
Received: June 16, 2016 | Published: September 13, 2016
Citation: Burge MR, Qualls CR, Kramer K, et al. Utility of subcutaneous octreotide in the early recovery from diabetic ketoacidosis in acutely ill type 1 diabetes patients. J Diabetes Metab Disord Control. 2016;3(5):99-103. DOI: 10.15406/jdmdc.2016.03.00079
Background: Diabetic Ketoacidosis (DKA) is a severe complication of type 1 diabetes. Previous studies have demonstrated that somatostatin improves recovery from experimentally induced DKA. We hypothesized that subcutaneous administration of long-acting somatostatin analogue would improve recovery from DKA in acutely ill patients admitted to the Medical Intensive Care Unit (MICU).
Study Methods: Ten subjects with C-peptide negative type 1diabetes and DKA were admitted to the ICU and randomized to receive octreotide 100mcg subcutaneously every 6hours for 24hours or matching placebo in addition to standard therapy in a double-blind, randomized pilot and feasibility trial. Outcome parameters were rates of change for serum bicarbonate, total serum ketone bodies, counter regulatory hormones, and length of stay in the MICU. Results are summarized in the data table below (Table 1).
Variable |
∆ Placebo Group (n=4) |
∆ Octreotide Group (n=6) |
p-value |
Total Ketones (mg/dl/hour) |
-349 ± 455 |
-696 ± 314 |
0.24 |
Bicarbonate (meq/l/hour) |
0.44 ± 0.46 |
0.90 ± 0.7 |
0.25 |
Free Fatty Acids (mmol/l/hour) |
0.003 ± 0.018 |
0.043 ± 0.075 |
0.26 |
Glucagon (pg/ml/hour) |
-0.6 ± 1.4 |
-2.9 ± 10.0 |
0.61 |
Growth Hormone (ng/ml/hour) |
-0.20 ± 1.0 |
-1.1 ± 1.5 |
0.29 |
Glucose (mg/dl/hour) |
-12.4 ± 19.5 |
-18.2 ± 14.0 |
0.63 |
Length of MICU stay (hours) |
28 ± 15 |
22 ± 14 |
0.52 |
Table 1 Outcome parameters were rates of change for serum bicarbonate, total serum ketone bodies, counter regulatory hormones, and length of stay in the MICU.
Conclusions: These data demonstrate that the subcutaneous administration of octreotide 100mcg every 6hours for the first 24hours of hospitalization does not improve the rate of recovery from DKA in acutely ill type 1 diabetics. Neither is the length of stay in the MICU reduced by octreotide therapy. Post-hoc power analyses suggests that 25-30 subjects per group would be required to achieve 90% power with alpha=0.05 given the differences in total ketones and bicarbonate that we observed.
Diabetic ketoacidosis is a serious and potentially deadly acute complication of type 1 diabetes. CDC surveillance data from 1996 indicates that approximately 120,000 individuals were diagnosed with DKA and that the mortality rate was 21 out of 100,000. Additionally, the number of deaths due to DKA has varied little in the last two decades.1 Standard treatment for DKA includes admission to an intensive care unit, frequent laboratory analysis, aggressive fluid hydration, continuous intravenous insulin administration, and supplementation with potassium, and bicarbonate, if needed. Novel interventions to treat DKA have not been introduced recently. DKA occurs as a result of either absolute or relative insulinopenia. In patients previously diagnosed with diabetes, stressors such as pregnancy, infection, trauma, or myocardial infarction can precipitate DKA. The physiologic stress response leads to release of several counter regulatory hormones, including cortisol, catecholamines, growth hormone, and glucagon, which are also gluconeogenic and/or glycogenolytic hormones.2
Glucagon in particular has been associated with development of DKA.3 For example, plasma glucagon concentrations correlate with the formation of ketone bodies.4 Additionally, previous studies have demonstrated that administration of somatostatin, an inhibitor of both glucagon and growth hormone, results in a more rapid reduction of acidosis compared to insulin alone.5 Unfortunately, the use of somatostatin in the treatment of DKA is problematic. The drug has a half-life of less than three seconds, thus necessitating a continuous intravenous infusion. Patients with DKA are often severely dehydrated, and standard treatment requires multiple intravenous infusions including insulin, fluid, glucose, and potassium. Finally, some patients occasionally require bicarbonate infusion, or intravenous antibiotics or other therapies to treat the precipitating cause. Intravenous access is thus limited for other necessary infusions. Octreotide, a somatostatin analogue, has a significantly longer half life than the parent hormone (60 to 110 minutes), and is 100% bioavailable by subcutaneous injection.6 As such it can be administered subcutaneously every 8 hours thus avoiding the pitfalls of somatostatin. Like somatostatin, octreotide inhibits both growth hormone and glucagon.7 Additionally, studies in rhesus monkeys have demonstrated that octreotide is 45 times more effective than somatostatin in suppressing Growth hormone secretion and 11 times more effective in suppressing Glucagon secretion.8
Diem et al have previously demonstrated that octreotide prevents ketogenesis following insulin withdrawal in patients with type 1 diabetes.9 Further, Yun et al, comparing the effects of octreotide vs octreotide plus insulin, showed improvement in ketonuria with the latter treatment in patients with DKA.10 However, to date no studies have determined the potential benefits of the addition of octreotide to standard care in the treatment of DKA. We hypothesized that administration of octreotide, subcutaneously for patients with DKA early in the course of hospitalization will hasten metabolic recovery and reduce the length of stay in the MICU.
Patient Demographics
The study protocol was approved by the University of New Mexico Research Review Committee. All patients gave written informed consent. Inclusion criteria included admission to the medical intensive care unit with a diagnosis of DKA and blood glucose > 300 mg/dl, urine ketones at least ++ positive, anion gap greater than 20, serum HCO3- less than 15 meq/L, and age between 18 and 70 years. Exclusion criteria were mental obtundation, systolic BP less than 70 mm Hg, documented severe underlying infection or sepsis, pre-existing end-stage renal failure or hepatic failure, concurrent diagnosis of another cause of metabolic acidosis, such as aspirin overdose or toxic ingestion, and current pregnancy or breastfeeding.
Study Protocol
Subjects who agreed to participate were randomly assigned to treatment with Octreotide, 100 mcg subcutaneously every 6 hours for a total of 4 doses, plus usual care or placebo, 1.0 ml in normal saline q 6 hours for four doses, plus usual care. Usual care consisted of intravenous insulin (0.1 u/kg./hr) and 0.9% saline (1-3 L/hr. for the first 4 hours, then 200-1000 cc/hr. depending on individual needs) Intravenous insulin infusion was adjusted on an individual basis. KCL was administered intravenously as necessary to maintain potassium balance. Intravenous infusion of 10% dextrose was initiated when the plasma glucose concentrate fell to 250 mg/dL. Blood samples to determine concentrations of glucose, glucagon, growth hormone, ketones, acetoacetate and beta-hydroxybutyrate, bicarbonate, potassium, free fatty acids, sodium, chloride, and triglycerides were drawn 10 seconds prior to intervention, at time 0, then every thirty minutes for 8 hours.
Additionally, patients were evaluated every four hours for determination of suitability for transfer out of the intensive care unit. Criteria for transfer included, resolution of metabolic acidosis (anion gap < 15 and/or serum bicarbonate > 20 meq/l), clinically acceptable glycemia and readiness to switch to subcutaneous insulin, readiness to resume oral feeding, and an absence of other severe medical problems necessitating continued MICU care.
Glucose samples were processed shortly after blood draw. All other samples were stored at –20 until processing including electrolytes, plasma glucose, glycerol, free fatty acids, glucagon, and growth hormone. Plasma glucose concentrations were determined by means of a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA). Plasma acetoacetate and beta-hydroxybutyrate concentrations were determined spectrocopically on the Cobas-Bio (Roche Analytical Intruments, Nutley, NJ) employing the methods of Mellanby and Williamson.11 Plasma free fatty acids levels were assayed enzymatically using the Wako kit procedure (Wako Chemical, Inc, Dallas, TX) adapted to the Cobas-Bio. Serum growth hormone and glucagon were determined by the clinical laboratory at Barnes Hospital (Washington University, St. Louis, MO) in combination with the Cobas-Bio.
Statistical analysis
The rate of DKA was compared between patients in the octreotide group and patients in the placebo group by means of an analysis of variance for repeated measures, where appropriate, followed by post-hoc two-tailed t-test. Length of MICU stay was compared between the study groups using the unpaired Student’s t-test. Data are reported as Mean ± Standard Deviation in the text and as Mean ± SEM in the data figures. A p value of less than 0.05 was considered statistically significant.
Ten subjects completed the study, nine males and one female, mean age, 34.4±4 years. Four subjects were assigned to placebo and 6 to octreotide. No adverse events were reported in either group.
Plasma glucose levels
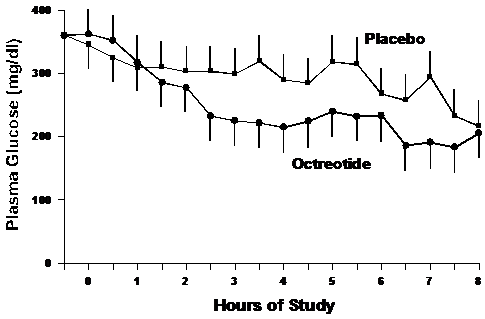
The plasma glucose levels are shown in Figure 1. Hyperglycemia was comparable between the groups initially. Glucose levels declined more rapidly in the octreotide group compared to placebo but did not reach statistical significance. Glucose levels declined at a greater degree in the octreotide group with a rate of 18.2±14.0 mg/dl/hour as compared to the placebo group (12.4±19.5 mg/dl/hour; p= 0.63).
Plasma Ketones
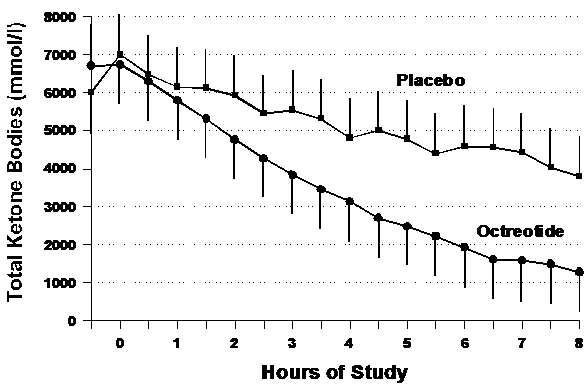
Figure 2 shows the effects of octreotide on total ketone body (TKB) concentrations. The rate of decline in total ketones was greater in the octreotide group (-696±314 mmol/l/hour) as compared to placebo (-349±455 mmol/l/hour) but did not reach statistical significance (p=0.24). However as with glucose, the AUC for ketones was lower in the octreotide group compared to placebo.
Serum Bicarbonate
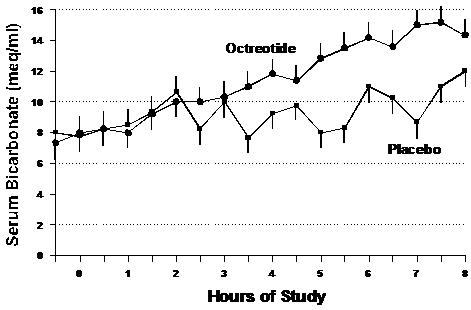
Figure 3 shows the mean rate of rise of serum bicarbonate for both groups. The bicarbonate rate of increase was 0.9±0.7 meq/l/hour for octreotide as compared with 0.44±0.46 meq/l/hour for placebo. Although a clear trend toward quicker normalization of bicarbonate is seen with octreotide, this data did not reach statistical significance (p=0.25).
Plasma Non-Esterified Fatty Acids (NEFA)
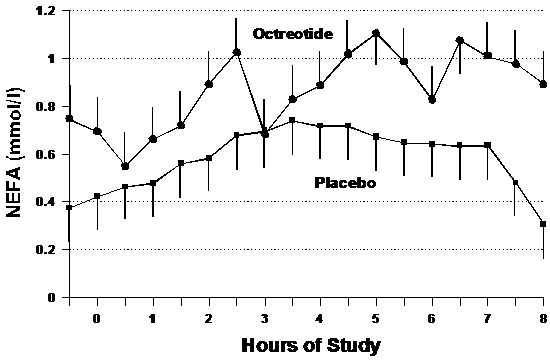
Figure 4 demonstrates the rise in plasma NEFA levels during treatment in both the placebo and octreotide groups. Although both groups demonstrate a general increase of NEFA, participants receiving placebo increased at a rate of 0.003±0.18 mmol/l/hour versus 0.90±0.07 mmol/l/hour in the octreotide group (p=0.26).
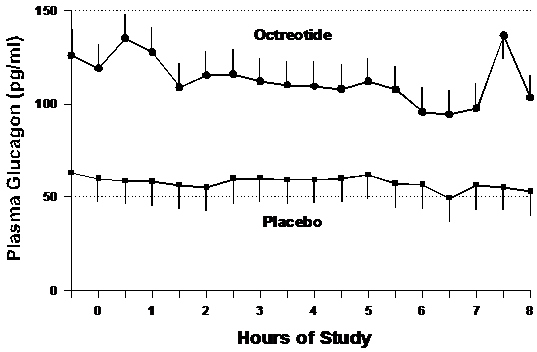
Glucagon
Glucagon concentrations were higher in the octreotide group at baseline (p<0.01), but as shown in Figure 5, the rate of decline of serum glucagon was not different between the groups. Glucagon levels declined at a slope of -0.6±1.4 pg/ml/hour in the placebo group and -2.9±10.0 pg/ml/hour in the octreotide group (p=0.61).
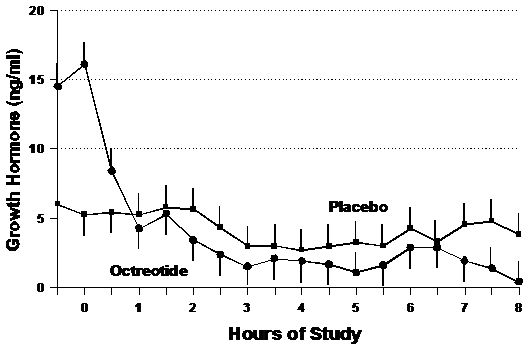
Growth hormone (GH)
Mean levels of the counter regulatory hormone, growth hormone (GH) are shown in Figure 6. Although GH was much higher initially in the octreotide study group, it declined sharply during the first hour and then declined at a rate of -1.1±1.5 ng/ml/hour, while the GH concentrations in the placebo group decreased at a steady rate of -0.20±1.0 ng/ml/hour over the entrie data collection period in the placebo (p=0.29).
Length of MICU stay (LOS)
Figure 7 indicates the average length of stay in the MICU. LOS was 22±14 hours for the octreotide group versus 28±15 hours for the placebo group (p=0.52).
In 1975, Gerich and colleagues showed that somatostatin infusion could prevent the development of ketoacidosis with type one diabetes patients deprived of insulin for 18 hours.12 This study demonstrated that the administration of somatostatin suppressed glucagon and prevented the increase of plasma glucose, 3-hydoxybutyric acid and non-esterified fatty acids. In 1976, however, Lundbaek et al.13 reported a failure of somatostatin to correct DKA in five patients with type 1 diabetes who underwent insulin withdrawal for 60-72 hours before somatostatin was administered without insulin.13 This produced a reduction in glucagon and growth hormone as expected, but also a fall in plasma glucose concentration during the four hour administration of somatostatin alone. Greco and colleagues demonstrated a benefit of somatostatin when administered as an adjunct to low dose insulin and fluid resuscitation in DKA patients in 1981.5 They found a significant increase in the rate of glucose decline in those treated with somatostatin as compared to insulin alone. The reduction was 73% in six hours for those treated with insulin alone and 80% in three hours in the group treated with somatostatin and insulin. Ketoacidosis resolved after 5 hours in the placebo group and resolved within 3 hours in the somatostatin group. Both plasma growth hormone and glucagon were suppressed to a greater degree with somatostatin and insulin, than insulin alone.
In 1982 somatostatin analog octreotide was formulated. It had both longer activity and increased potency. It is 45 times more effective at suppressing growth hormone and 11 times more effective at suppressing glucagon than native somatostatin in rhesus monkeys. Diem et al clearly showed in 1991 that administration of octreotide could prevent the development of ketoacidosis.9 In that study, seven type 1 diabetic subjects underwent insulin withdrawal for 9 hours on two different occasions at least three weeks apart. During one of these occasions, 50 micrograms of octreotide was given 9 hours prior to insulin withdrawal and every three hours until insulin was restarted. Baseline plasma glucose, free fatty acids, beta-hydroxybutyrate, acetoacetate, and glucagon levels, as well as arterial pH and bicarbonate, were not significantly different from prior to insulin withdrawal. The rise in ketone body production, however, was significantly reduced when the subjects were given octreotide as compared with patients who did not receive octreotide. Arterial pH and serum bicarbonate recovery both reached statistical significance, and glucagon concentrations were significantly depressed with administration of octreotide, as expected.
In 1999, Yun and colleagues utilized octreotide in the setting of established ketoacidosis.10 23 patients who were admitted to the hospital with a diagnosis of DKA were randomized to receive either low dose insulin alone or low dose insulin plus octreotide. Seven patients were treated with somatostatin 50 micrograms every 6 hours with standard fluid replacement and insulin infusion. 16 patients received standard therapy of fluid replacement and insulin alone. Three endpoints were studied, including time for correction of hyperglycemia, correction of acidosis, and resolution of ketonuria. Although a trend toward shortened time for correction was seen in all three outcome parameters, only resolution of ketonuria was statistically significant with recovery rates of 38±32 hours in the octreotide group and and 68±26 hours in the placebo group (p=0.005). Hyperglycemia correction time was 8.9±3.2 hours with octreotide versus 18.8±13.2 hours with placebo (p>0.05).
These studies and others have shown that increased levels of counter regulatory hormones clearly contribute to the pathogenesis of DKA, and their suppression by somatostatin has proven to shorten recovery from ketoacidosis. However, the cumbersome use of continuous intravenous somatostatin has limited its use as a practical adjunctive treatment to standard low dose insulin therapy and fluid resuscitation. The improved pharmacokinetics and more potent characteristics of longer-acting octreotide make it an ideal adjunct in theory in DKA. However, no study has definitively shown that octreotide has the effect of reversing DKA in patients who are acutely ill. This pilot study attempts to address this deficiency. Although our preliminary results show that administration of octreotide did not significantly alter recovery from DKA, several important points deserve further discussion. First, low dose insulin and fluid resuscitation alone produced a precipitous improvement in all the parameters studied.14 This fact may have somewhat obscured the positive effects that adjunctive octreotide may have on the recovery of DKA. Also, many of the studies supporting the use of somatostatin in DKA have been performed using experimentally induced DKA, this paradigm may not include all the factors seen in patients presenting with real-life DKA, including alcohol ingestion, sepsis, malnutrition, and severe dehydration. Further data must therefore be collected on ill patients presenting with DKA to establish the most effective clinical use of octreotide prior to any consideration of widespread use. Of course, the underlying cause of ketosis may also influence recovery from DKA, and this factor was not accounted for in our study design.
Limitations of this study clearly include the small sample size. Although a trend toward greater improvement was seen in all parameters among patients who received octreotide, none of our findings were statistically significantly different from those who received placebo. Post-hoc power analysis indicates that 26 patients are required in each study group to achieve 90% power to detect an improved rate of recovery from DKA as reflected in the serum bicarbonate concentration with and alpha level of 0.05. As such, our current findings very likely reflect a Type II statistical error, and further study of octreotide used in the setting of acute DKA should be pursued.
None.
Author declares that there is no conflict of interest.

©2016 Burge, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.