Journal of
eISSN: 2374-6947


Research Article Volume 5 Issue 6
Laboratory of Physiology, Pharmacology and Pharmacopoeia, UFR-Science of Nature, University in Abidjan, Côte d’Ivoire
Correspondence: Monteomo Gnate Francois, Laboratory of Physiology, Pharmacology and Pharmacopoeia, UFR-Science of Nature, University in Abidjan, Côte d’Ivoire
Received: December 13, 2018 | Published: December 19, 2018
Citation: Monteomo GF, Kamagate A, Yapo AP. Effects of metabolism syndrome on blood cells to Wistar rats. J Diabetes Metab Disord Control. 2018;5(6):222-225. DOI: 10.15406/jdmdc.2018.05.00170
This investigation is concentrated on how hematological markers would change in pig fat induced metabolism syndrome (MS) in adult Wistar rats. The objective of the current work was to examine the effect of diet-induced metabolic syndrome on hematological parameters. Metabolic syndrome was induced by feeding rats with diet supplemented to 10% (LF group) and 30% (HF group) pig fat and the control rats were fed normal diet (ND) for 9 weeks. After the study period, White Blood Cell (WBC), Red Blood Cell (RBC), hematocrit (HCT), Hemoglobin, platelet (PLT), lymphocytes, monocytes, Neutrophils, Mean Corpuscular Hemoglobin Concentration (MCHC), Mean Corpuscular Volume (MCV) were measured using BC-30s hematology analyzer.
The results showed that hematocrit, RBC and hemoglobin levels were significantly decreased in metabolic syndrome than in control rats respectively (37.24±10.3 vs 33.16±9.5%; 7.08±3.2 vs 6.63±2.8x106/μL and 13.36±1.3 vs 11.85±1.2g/L; p< 0.05). MCHC and platelet were also not elevated in metabolic syndrome than in control rats respectively (31.5±8.2 vs 45.3±10.4 and 356.6±92.2g/dl vs 485.2±89.4x106μL; p<0.05). However, erythrocyte indices as MCV didn’t showed statistically significant variations among the LF and control rats (56.08±2.01 vs 55.4±2.8fl; p<0.05). MS appeared to significantly decrease to white blood cell concentration, lymphocytes and neutrophils which probably reduced the immune defense mechanism. The findings demonstrated that MS caused significant undesirable alterations in hematological parameters in rats and it possible to suspect a coexistence between anemia and the metabolic syndrome in this animal model. In conclusion, the rats induced fat metabolism syndrome affected hematocrit and hemoglobin levels and it possible to suspect a coexistence between metabolic syndrome and the anemia in this animal model.
Keywords: Hematogical parameters, Fat diet, Metabolism syndrome, Wistar rat
Metabolic syndrome (MS) is a multiplex risk factors for cardiovascular disease and type 2 diabetes mellitus. The defining clinical criteria of MS include central obesity, dyslipidaemia, hypertension and glucose intolerance.1 This epidemic affects about 35% and 50% of the adult population respectively to United States and Northern Europe.2 The prevalence of MS varies by 4.4 % in Côte d'Ivoire.3
According to Etim,4 the examination of blood provides the opportunity to clinically investigate the nutritional, physiological and pathological status of an individual or an animal. The number of the blood count also called blood count is the most prescribed biological examination for all pathologies. It provides information about blood cells that help maintain the integrity of the body. This examination includes the counting of all cells or figured elements of the blood and studies certain particular parameters of the blood. It is a quantitative and qualitative study that is to say it allows to determine the number of red blood cells accompanied by parameters allowing to characterize the erythrocyte population, leucocytes of blood and the detection of other abnormal cells. Among the erythrocyte indices, the mean corpuscular volume (MCV) which accounts for the size of red blood cells, is a blood parameter thus helps to establish the etiological diagnosis of anemia. MCV defines the size of the red blood cells and the mean corpuscular hemoglobin (MCH) quantifies the amount of hemoglobin per red blood cell. The mean corpuscular hemoglobin concentration (MCHC) indicates the amount of hemoglobin per unit volume. In contrast to MCH, MCHC correlates the hemoglobin content with the volume of the cell.5 According to the size of the red cell, anemias are classified, as being normocytic (normal MCV), macrocytic (increased MCV), or microcytic (decreased MCV) while the MCHC is blood parameters for identifying normo and hypochromic anemias.5
The numbers of total leukocytes, neutrophils and lymphocytes were elevated levels increased in accordance with the numbers of delected metabolic components.6 Hematocrit (HCT) was reported to be positively associated with insulin resistance, which is the basic pathogenesis for MS,7 and elevated erythrocyte parameters were confirmed to be associated with MS or its components, suggesting that erythrocyte parameters might be a potential predictor for risk of MS.8
Increasing studies have reported that erythrocyte parameters, including red blood cells (RBCs), haematocrit, haemoglobin (Hb) and red blood cell distribution width (RDW), are associated with metabolic syndrome (MS). A number of these studies have revealed that metabolic syndrome could exert diverse effects on haematological indices of animals.9 and humans.10‒12
Although various cross-sectional studies have shown that erythrocyte parameters, including red blood cell, hemoglobin and hematocrit, were linked with metabolic syndrome (MS), few experimental studies have been used to confirm their relationship. The only the combination of sugar and fat, present in beverage and diet was found to result in the development of metabolic syndrome and liver vascular complications in rats.13
This work is based on the use of an experimental model of nutritional metabolic syndrome, the Wistar rat is subjected to a normal diet supplemented with low dose of 10% (LF) and high dose of 30% (HF) pig fat to follow some changes in hematological parameters.
Animal material and livestock
The experimental protocol consisted of 21 rats mixed in 3 groups of 7, as well as Rattus norvegicus of Wistar strain composed of males and females, of the pet shop of the physiology, pharmacology and pharmacopoeia laboratory of Nangui Abrogoua University, Abidjan. Rats aged 8 to 9 weeks and with a homogeneous mean body weight of 110.60±7.5 g are fed with standard diet (FACI®, Abidjan, Côte d’Ivoire) for control rat or grains added to 10% (LF) and 30% (HF) pig fat for experimental rats for 9 weeks (63 days). The breeding is done in a lighted room 12hours a day, and whose temperature is kept constant (22 to 23°C) in an enclosure equipped with an air conditioner (Smart, Canada). The animals have free access to food and water and are weighed once a weeks.
Blood sampling
Blood samples were taken in healthy rats on days 0 and apparently healthy at the experience end. The rats are anesthetized with a solution of ether soaked in cotton after 12 hours of fasting. The blood collected by ponctures of the retro-orbital sinus is recovered in EDTA tubes in order to count the number and see the shape of the blood cells the same day of sampling.
Determination of hematological parameters
The hematological parameters are determined quantitatively automatically by a BC-30s hematology analyzer. This device consists of the main unit, reagents, controls and calibrators. The analyzer uses the electrical impedance method to determine the count and size distribution of RBC, WBC and PLT; it uses the colorimetric method to determine HGB. From the aforementioned data, the analyzer calculates other parameters.14
Blood smear
A droplet of blood is deposited on a glass slide used to prepare the blood smear. Staining allows the identification of leukocytes by their specific characteristics highlighted by a suitable dye. Smear staining was performed with a May-Grunwald-Giesma solution, based on the successive use of two dyes: May-Grunwald and Giesma. The first fixes the smear by its methyl alcohol and colors the acidophilic elements and specific granulations of the leucocytes. The second one colors especially the nuclei and the azurophilous parts. The smear is examined under an optical microscope using the objective (x 100).
Statistical analyses
The statistical analysis of the data is done with the GraphPad Prism 5.01 software (San Diego, California, USA). Whenever a significant difference (p<0.05) is revealed, the ANOVA test is completed with Tukey's post ANOVA test to identify the variable (s) with very significant differences from the values witnesses (Table 1). The difference between two means is considered significant if p<0.05, highly significant (Figure 1).
| Initial values (0 Week) | Final values (9 Weeks) | |||||
| Physiological parameters | Control | 10% LF | 30% HF | Control | 10% LF | 30% HF |
| WBC (103/μl) | 11.09±2.4a | 11.02±2.3a | 10.95±1.9a | 11.3±2.5a | 15.1±2.7b | 12.1±2.1b |
| RBC (106/μl) | 7.67±2.9a | 7.3±3.1a | 7.3±2.8a | 7.086±3.2a | 6.84±2.9b | 6.63±2.8b |
| Hb (g/dl) | 13.2±1.02a | 12.89±1.2a | 12.8±1.01a | 13.36±1.3a | 13.16±1.5a | 11.8±1.2b |
| HCT (%) | 37.8±9.4a | 36.95±9.7a | 36.95±8.6a | 37.2± 10.3a | 33.1±9.5b | 34.5±8.7b |
| MCV (fl) | 55.9 ±1.9a | 54.8±2.1a | 54.7±2.06a | 56.08±2.1a | 55.2±2.4a | 55.4±2.8a |
| MCH (pg) | 24.7±9.4a | 24.1±10.1a | 23.8±9.6a | 25.7±11.7a | 25.1±10.3a | 17.3±9.8b |
| MCHC (g/dl) | 44.8±9.2a | 45.1± 8.6a | 44.20± 9.5a | 45.3±10.4a | 44.54±9.8a | 31.5±8.2b |
| PLT (106/μl) | 358,3±86a | 356,6±81a | 359±86.4a | 356.6±92.2a | 485.2±89.4b | 294.5±96b |
| NEU (%) | 1.9±0.39a | 1.95±0.4a | 1.85±0.34a | 1.8±0.64a | 1.9±0.49b | 1.02±0.37b |
| LYM (%) | 7.9±0.37a | 7.2±0.2a | 7.01±0.35a | 8.02±0.28a | 11.4±0.2b | 8.5±0.24b |
| Mo (%) | 1.29±0.6a | 1.19±0.4a | 1.14±0.6a | 1.36±0.89a | 1.8±0.75b | 1.55±0.6b |
| HDL-C,mg/dl | 90.5±5.4a | 78.5±5.7a | 91.1±4.8a | 93.5±4.1a | 61±4.9b | 54.9±5b |
| LDL-C,mg/dl | 92.5±4.7a | 90.1±4.1a | 79.5±4.3a | 90.4±4.2a | 96.4±9.3b | 112.8±8b |
| TG,mg/dl | 134.2±1.4a | 131.5±1.6a | 138 ± 1.48a | 136.2±4.8a | 142±5.3b | 148.86±4b |
Table 1 Means assigned the same letter on the same line are not significantly different at the 0.05 threshold According to the Tukey test. (n=7): number of animals per group; p<0.05; (*)=significant The experimental rats with low dose of 10% (LF) and high dose of 30% (HF) pig fat diet were induced metabolic syndrome
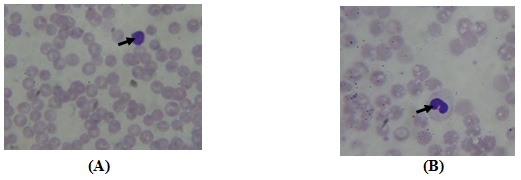
Figure 1 Photomicrographs of metabolism syndrome diet of 30% rats blood showing the presence of Lymphocyte (A) and Monocyte (B). (10 x; MGG).
The experimental rats with low dose of 10% (LF) and high dose of 30% (HF) pig fat diet were induced metabolic syndrome. The Control rats (Control) were fed normal diet. RBC, red blood cell; WBC, White blood cell; Hb, haemoglobin; HCT, haematocrit; PLT, platelet; MCV, Mean Corpuscular volume ; MCH, Mean Corpuscular Hemoglobin; MCHC, Mean Corpuscular Hemoglobin Concentration; Neutrophils, NEU; Lymphocytes, LYM; Monocytes, Mo. HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; Triglycerides, TG.
For 9 weeks the different diets were followed and at the end of the experiment the blood is taken for cell counting (NFS) and hemoglobin determination. The values of hematocrit (HCT), Red blood cell (RBC) count and hemoglobin concentration significantly decreased in rats metabolic syndrome than in control rats respectively (37.24±10.3 vs 33.16±9,5%; 7.08±3.2 vs 6.63±2.8x106/μL and 13.36±1.3 vs 11.85±1.2g/L) in contrast to the high values of these erythrocyte parameters in clinical cases.15 which are (42.27±4.09 vs 40.68± 3.63%; 4.99±0.8 vs 4.53±0.5x102/L and 147.11±12.57 vs 139.02±12.68g/L). In fact, the reduction in erythrocyte parameters observed in our trial could be due to the coexistence metabolic syndrome and anemia in the same individual.16 Individual with high serum ferritin were found to have higher risk of the metabolic syndrome and combined anaemia and the metabolic syndrome,16 this last case is seen in our experience. Other findings, hemoglobin levels was positively associated with MS in animal and human. Young rats with all the symptoms of metabolic syndrome induced by a fat-enriched diet (60% Kcal) experienced a significant increase in hemoglobin compared to control rats,9 probably due to the absence of anemia. In this last case, association between MS and higher hemoglobin levels in the elderly indicating that current dietetic recommendations.10
Platelet count is one of the most important screen tests of platelet function. A decrease in the circulating platelets less than 50% of the normal value will cause bleeding.17 The reduction observed in the platelet counts in HF rats than control rats (356.6±92.2 vs 294,5±96x106/μl; p<0.05) may indicate possible effects on blood clotting in the fat metabolic syndrome rat. However, the increased platelet counts in LF rats than control (240,5 vs and 485.6±89.4 vs 294,5±96x106/μl; p<0.05), high a platelet count sometimes indicated inflammatory or infectious disease and liver disease in this syndrome.18 Erythrocyte indices don’t define the cause of anemia, but they may be helpful during the diagnostic workup. A significant decrease in the number of red blood cells, MCHC and MCH is observed with metabolic syndrome HF rats compared to the control respectively (7.08±3.02 vs 6.63±2.8x103/μL; 45.3±10.4 vs 31.5±8.2g/dl and 25.7±11.7 vs 17.35± 9.8pg; P<0.05). The MCV value did not have a significant difference in the whole of the rats and suggesting a hypochromic normocytic anemia in these animals. This result corroborated with that of rats having lower hemoglobin concentration and hematocrit after consuming (21.1%) fat diets,19 and had similary to iron deficiency aneamia.20
The SM led to a significant change in the blood count while the number of leukocytes increased significantly in LF and HF rats than the control rats (15.14±2.7 and 12.18±2.1 vs 11.3±02.5; P<0.05) at the end of experience. In fact, high WBC count is strongly associated with metabolic syndrome in rat,21 and human.22,23 White blood cell count was significantly positively correlated with body mass index, the expression of an insulin-resistant state or involvement of the MS (Nakanishi, 2004; Park, 2009). And, an increase in the WBC count with the MS could be due to the presence of lymphocytes and monocytes in this inflammatory reaction (Figs: lymphocyte and Monocyte).
The increase in leukocytes can be characterized by an increase in the number of monocytes in HF rats compared to controls (1.36±0.89 vs 1.55±0.64; P<0.05) and lymphocytes in LF rats compared to controls (8.20±0.28 vs 11.4±0.21; P<0.05). Monocytes patrol the circulation in search of diseased or senescent RBC because HFD-induced proinflammatory responses and RBC alterations. These RBC alteration may be relevant to the mechanisms of atherosclerosis in the setting of metabolism syndrome in mice and human.21,24
Neutrophils derived from bone marrow colony-forming unit granulocyte/macrophage progenitors dominate the early stages of inflammation and set the stage for tissue infiltration by macrophages. By contrast with macrophages, neutrophils have classically received only little attention in MS because of low neutrophil count noted in LF rat than the control rat (1.8±0.64 vs 1.02±0.37 %) could be a deterioration of the immune system. In other study of high fructose-induced MS, significantly increased neutrophil phagocytic activity was observed in early stage of high-fructose diet-induced MS to rats compared with the control group (154.6±12.77 vs 206.5±14.83; P<0.05) after activation with opsonized E. coli; the results of this study provide evidence a modification in the production, function of circulating neutrophils and a promotion of their adipose tissue infiltration at an early stage of MS.25
In total, an association between inflammation and the metabolic syndrome has been found.26 while increase of the WBC, monocytes and neutrophils.
Changes in hematology parameters in the metabolic syndrome were dominated by hypochromic normocytic anemia corresponding to a decrease in erythrocyte hemoglobin, hematocrit and reticulocyte contents (MCH, MCHC) and normal value of MCV. This makes it possible to suspect a coexistence between metabolic syndrome and the anemia in this animal model. This anemia seems infectious taking into account the decrease in the number of neutrophils and an increase in lymphocytes and inflammatory based to the presence of monocytes.
The author would like to thank the staff of Laboratory of Physiology, Pharmacology and Pharmacopoeia, NANGUI ABROGOUA University to provide assistance to testing on laboratory animals.
The author declares no conflict of interest.

©2018 Monteomo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.