Journal of
eISSN: 2374-6947


Research Article Volume 4 Issue 5
1Program in Clinical and Experimental Therapeutics, University of Georgia, USA
2Charlie Norwood Veterans Affairs Medical Center, USA
3Augusta Biomedical Research Foundation, USA
Correspondence: AB El-Remessy, PhD, RPh, FAHA Augusta Biomedical Research Foundation Charlie Norwood Veterans Affairs Medical Center Downtown 6B-152, Augusta, Georgia 30912, USA, Tel +1 706 733 0188, Fax +1 706 823 2268
Received: July 24, 2017 | Published: December 21, 2017
Citation: Mohamed R, Shanab AY, Remessy ABE. Deletion of the neurotrophin receptor p75 ntr prevents diabetes-induced retinal acellular capillaries in streptozotocin-induced mouse diabetic model. J Diabetes Metab Disord Control. 2017;4(6):163-169. DOI: 10.15406/jdmdc.2017.04.00129
Diabetic retinopathy is characterized by early stage of retinal neuro-inflammation that triggers development of acellular capillaries and a late stage of pathological neovascularization. Due to limited treatment options, there is a pressing need to develop new therapeutics. Our group discovered that diabetes-impaired processing of the nerve growth factor precursor (proNGF) resulting in its accumulation and its receptor p75NTR. Here, we examine the protective effects of modulating p75NTR in experimental model of diabetic retinopathy. Diabetes was induced using streptozotocin in both wild type (WT) and p75NTR knockout (p75KO) mice. Retinal inflammation and microvascular dysfunction were assessed. Western blot analysis was performed to assess expression of apoptotic and inflammatory markers and levels of the neurotrophin, p75NTR and ephrin-B2. Deletion of p75NTR did not alter body weight or diabetes status compared to WT mice. In WT-mice, diabetes triggered retinal inflammation, significant decrease in pericyte count and marked increase in development of occluded (acellular) capillary formation after 24weeks. Deletion of p75NTR prevented acellular capillary, restored pericyte count, and inhibited the retinal Ephrin-B2, activation of the stress-kinase JNK and apoptotic marker cleaved caspase-3 in the diabetic retina. Deletion of p75NTR reduced retinal inflammation, and proNGF expression. These effects coincided with increased NGF level and TrkA activation in the diabetic retina. Targeting p75NTR using genetic approach protected the retina from the impact of long-term diabetes in mediating microvascular degeneration and maintains the balance of NGF/proNGF level. Together, these results provide rationale that targeting p75NTR may offer novel and effective therapeutic strategy to combat diabetic retinopathy.
Keywords: diabetic retinopathy, retina, p75ntr, neurotrophin, diabetes, acellular capillary, pericyte, apoptosis
ARVO, association for research on vision and ophthalmology; BRB, blood-retina barrier; DR, diabetic retinopathy; GAPDH, glyceraldehydes 3-phosphate dehydrogenase; IL-1β, interleukin-1 beta; JNK, c-june kinase; KO, knock out; NF k B, nuclear factor kappa beta; NG2, neuroglial antigen-2; NGF, nerve growth factor, proNGF, proform of nerve growth factor; PASH, periodic acid-schiff and hematoxylin; p75NTR, neurotrophin receptor p75; STZ, streptozotocin; TrkA, tyrosine kinase receptor-A; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; WT, wild type
Diabetic retinopathy (DR), the leading cause of blindness among US working age adults, is characterized by early inflammation, breakdown of blood-retina barrier (BRB), neurovascular degenerative changes.1,2 The microvascular degeneration is a slow process that involves apoptosis of endothelial cells and pericyte loss that lead to development of occluded (acellular) capillary formation resulting in retinal ischemia.3 Development of clinically significant ischemia switch DR to the proliferative stage, where upregulation of pro-angiogenic factors including vascular endothelial growth factor (VEGF) resulting in retinal neovascularization and eventually blindness.4,5 Current therapeutic options, including photocoagulation, vitrectomy, and repeated intravitreal anti-VEGF injections, are invasive and limited by considerable side effects.6 Moreover, these treatments do not address retinal ischemia and may accelerate neurodegeneration. Therefore, there is a great need for novel therapeutic targets that will preserve retinal neurons and vasculature.
Recent findings indicate that diabetes-induced alteration in growth factors and neurotrophin expression play pivotal roles in diabetic retina.7,8 The p75NTR is a multifunctional membrane receptor belongs to tumor necrosis factor (TNF) receptor superfamily, originally identified as a receptor for nerve growth factor (NGF). The upregulation of p75NTR is associated with many vascular inflammatory diseases including DR.9 Our group showed that diabetes impairs the processing of the NGF precursor (proNGF) to the mature legend NGF resulting in imbalance of proNGF/NGF ratio.10-13 The imbalance of proNGF/NGF during diabetes was associated with alteration and expression of TrkA and p75NTR receptors (reviewed in9-14). The impaired TrkA activity was associated with upregulation of p75NTR receptor, resulting in favoring activation of cell death pathway, neuro degeneration and BRB breakdown in the diabetic retina.11,15,16 Genetic deletion of p75NTR prevented diabetes-induced short-term neurodegeneration, inflammation and BRB breakdown.11 Prior studies using stable over expression of proNGF showed that upregulated p75NTR expression coincided with endothelial cell death17 and pericyte cell death.18 Other reports showed that short-term diabetes triggered p75NTR and pericyte death.16,19 However, the effects of modulating p75NTR expression on the long term effects of diabetes-induced retinal microvascular degeneration remain unknown. Such knowledge is critical in developing new therapies for treating DR. The aim of this study is to examine the protective effects of modulating p75NTR expression using genetic approach in the diabetic retina at a time point well-accepted to detect meaningful changes in retina vasculature.20,21 The current study demonstrated that modulating p75NTR expression inhibited formation of occluded capillaries and pericyte loss, hall mark of retinal ischemia. The study also revealed the impact of deleting p75NTR on diabetes-induced apoptosis and inflammatory pathways.
Animals
All animal procedures were performed in accordance with Association for Research in Vision and Ophthalmology (ARVO) statement for use of animals in ophthalmic and vision research, and Charlie Norwood VA Medical Center Animal Care and Use Committee (ACORP#16–01–088).
Induction of diabetes
Male 8-week old littermates of wild-type and p75NTR knockout mice (~25g) from our colony with founders originally purchased from Jackson laboratory (Bar Harbor, ME, USA). Mice were randomly assigned into four groups; WT-control, WT-diabetic (WT-STZ), p75KO control (p75KO), p75KO diabetic (p75KO-STZ). Mice were rendered diabetic by 5-consecutive intraperitoneal injections of streptozotocin, 50mg/kg body weight, (Sigma, St. Louis, MO) in 0.01-mol/l citrate buffer adjusted to pH 4.5. Blood was sampled from tail vein and glucose levels were measured with a glucose meter (ReliOn Ultima, Alameda, CA) every 2-weeks. Body weight was recorded and 1U of insulin was administered subcutaneously to prevent animals from losing more than 15% of their body weight. Hyperglycemia was defined by blood glucose level > 250 mg/dL and was confirmed by measuring HbA1C (Sigma Aldrich, MO) every 12 weeks. Animals were sacrificed after 24-weeks and eyes were enucleated for further analysis.
Isolation of retinal vasculature
The retinal vasculature was isolated as described previously.17,22 Freshly enucleated eyes were fixed with 2% paraformaldehyde overnight. Retinal cups were dissected, washed in PBS, then incubated with 3% Difco-trypsin 250 (BD Biosciences) in 25mM Tris buffer, pH 8, at 37°C for 2 hrs. Vitreous and nonvascular cells were gently removed from the vasculature that was soaked in several washes of 0.5% Triton X-100 to remove the neuronal retina.
Determination of occluded (acellular) capillaries
Trypsin-digested retinas were stained with periodic acid-Schiff and hematoxylin (PASH). Numbers of acellular capillaries were quantified in six-different areas of the mid-retina under the microscope (20X) in a masked manner by two different researchers. Acellular capillaries were identified as capillary-sized blood vessel tubes with no nuclei along their length. Trypsin-digested retinas were stained with anti-collagen IV polyclonal antibody and CD31 monoclonal antibody followed by secondary antibodies to visualize the signal.
Pericyte count
The retinal vasculature was isolated as described previously.23,24 Trypsin-digested retinas were stained with anti-NG2 antibody. Nuclei of pericytes were identified as being darker and smaller than endothelial cell nuclei and lie in the outer aspect of the capillary wall. Six animals were used for each group and the number of acellular capillaries were counted in 10 fields of six different areas of the mid-retina and calculated as the number/mm2 of retinal area. Pericyte counts were quantified in six-different fields of the retina under the microscope (20X) in a masked manner by two different researchers.
Western blot assay
Retinas samples (30μg protein) were homogenized in RIPA buffer and separated by SDS-PAGE.14 Antibodies were obtained commercially as listed in (Table 1). Antibodies for p75NTR was kindly provided by Dr. Bruce Carter (Dept. of Biochemistry, Vanderbilt University). Membranes were re-probed with β -actin and GAPDH (Sigma-Aldrich, St. Louis, MO) to confirm equal loading. The primary antibody was detected using horseradish peroxidase-conjugated sheep anti-rabbit antibody (GE Healthcare, Piscataway, NJ) and enhanced chemiluminescence (Pierce, Thermo scientific). Band intensity was quantified using densitometry software (alphEaseFC) and normalized to β-actin.
Antibodies |
Catalog Number |
Company |
Anti-VEGF |
Cat.# 676472 |
Millipore, Billerica, MA |
Anti-EFNB2 |
Cat #: PA5-19361 |
|
Anti-proNGF |
Cat.# N-250 |
Alomone Labs, Jerusalem, Israel |
Anti-NGF |
Cat.# AN-240 |
|
Anti-IL-1β |
Cat.# ab9722 |
Abcam, Cambridge, MA |
Anti-GAPDH |
Cat.# G8795 |
Sigma-Aldrich, St. Louis, MO |
Anti-b-actin |
Cat.# A2228 |
|
Anti-JNK |
Cat.# sc-7345 |
Santa Cruz Biotechnology, Dallas, Texas |
Anti-p-JNK |
Cat.# sc-293136 |
|
Cleaved-PARP |
Cat.# 5625 |
Cell Signaling Technology, Danvers, MA |
Anti-p75NTR |
## |
Dr. Bruce Carter (Dept. of Biochemistry, Vanderbilt University |
Table 1 A summary of sources of antibodies used to detect protein expression using Western Blot
GAPDH: Glyceraldehyde 3-phosphate Dehydrogenase; IL-1 β: Interleukin-1 beta; JNKC: June Kinase; NGF: Nerve Growth Factor; proNGF: Proform of Nerve Growth Factor; p75NTR: Neurotrophin Receptor p75; TrkA: Tyrosine Kinase Receptor-A; VEGF: Vascular Endothelial Growth Factor
Statistical analysis
Results are expressed as mean ±SD and the data were evaluated for normality and appropriate transformations were used when necessary. Data was processed for statistical analysis with two-way ANOVA followed by Bonferroni post-tests were used to adjust for the multiple comparisons to assess significant effects using Prism (Graphpad-Ver.6). Significance was defined as P<0.05.
Deletion of p75NTR does not alter body weight or blood glucose level
Diabetes induced significant increases in blood glucose levels associated with a progressive, significant, decrease in body weight in both WT and p75KO. Hyperglycemia was maintained in both WT and p75KO over 24-weeks (Figure 1). At 26-weeks, analysis of HbA1C showed significant increase in both diabetic WT and p75NTR compared to corresponding non-diabetic controls (Figure 1). These data lend further support to prior findings that deletion of p75NTR did not influence the diabetic state in p75KO compared to WT mice.11,23

Figure 1 Genetic deletion of p75NTR did not alter body weight or blood glucose in control and diabetic mice. Control animals exhibited a significant increase in body weight, but diabetic animals did not. B, C Blood glucose level was measured at various times after STZ administration. There was no increase in blood levels in control animals. A significant rise in blood glucose and HbA1c was seen in STZ-administered animals. There was no significant difference in blood glucose between diabetic WT and p75KO animals.
Diabetes increases retinal p75NTR and inflammation in WT but not in p75KO mice
Diabetes caused increase in retinal p75NTR expression in WT mice compared to controls, whereas p75KO retina showed no band for p75 confirming the genetic deletion (Figure 1). These results are in agreement with prior findings in short term diabetic animals.11,15,26 Next, we determined the impact of deletion of p75NTR on the long term diabetes-induced inflammation. Inflammation and IL-1β have been established as central player in the pathology of DR.27-29 Consistent with our previous report, genetic deletion of p75NTR suppressed long-term diabetes-induced IL-1β inflammation (Figure 2). We and others have shown that modulating p75NTR expression or activity significantly reduced TNF-α expression by inhibiting NF B activation in retina of short term diabetic animals.10,11,16 and in response to stable proNGF over expression.11,30

Figure 2 Genetic deletion of p75NTR suppressed diabetes-induced inflammation. (A): Western blot showed that diabetes significantly increasing the p75NTR expression in retina compared to WT control. (A&C): Diabetes induced significant activation of IL1beta in WT diabetic compared to controls. Genetic deletion of p75NTR suppressed inflammatory marker in the diabetic retina compared to respective controls.
P < 0.005 versus control animals.
Deletion of p75NTR ameliorated diabetes-induced retinal proNGF levels and restored NGF and TrkA activation
We previously reported that diabetes impairs maturation of NGF leading to significant increases in proNGF levels in experimental and clinical samples.12,13 In this study, we examined the long-term effects of diabetes and to what extent p75NTR deletion modulates diabetes-induced proNGF accumulation. The results showed significant accumulation of proNGF occurred in WT-STZ retinas (1.9-fold) compared to WT-controls (Figure 3). Interestingly, deletion of p75NTR prevented diabetes-induced increases in proNGF levels (Figure 3). These results support prior finding that down regulation of p75NTR prevent the increase in proNGF levels.11,17 Diabetes also significantly decreased (~50%) NGF levels in WT-STZ compared to WT-controls (Figure 3). Deletion of p p75NTR prevented diabetes-impaired NGF levels and prevented increases in proNGF levels, thus restoring the balance between NGF and proNGF in the diabetic retina. Diabetes caused significant decrease in TrkA activation (Y495) compared to controls in WT mice (Figure 3). In p75KO, improved ratio of NGF to proNGF was paralleled with restoration of the TrkA phosphorylation in diabetic retina compared to controls. This observation is critical in the light of recent findings that proNGF can switch between neurotropic and apoptotic activity in response to changes in TrkA receptor levels.31 Our results lend further support to the interplay between p75NTR expression and TrkA activation previously demonstrated in retinal endothelial cells17,32 and in hypoxia-induced retinal neovascularization model.33 However, this is the first report that shows that deletion of p75NTR can restore NGF and TrkA activation in the diabetic animals.

Figure 3 Genetic deletion of p75NTR suppressed diabetes-induced proNGF and restore NGF and TrkA activation. (A-E): Diabetes induced significant increases in proNGF and decreased the NGF level compared to controls. Genetic deletion of p75NTR suppressed the imbalance of proNGF/NGF and restores the NGF (C) and TrkA level (E) in diabetic retina compared to controls.
P < 0.05 versus control animals.
Deletion of p75NTR mitigated diabetes-induced occluded (acellular) capillaries in the retina
Development of retinal ischemia is a slow process that involves apoptosis of endothelial cells and pericyte loss.3,9 Quantification of occluded (acellular) capillaries and the number of pericytes are well-accepted surrogate markers for diabetes-induced retinal ischemia in rodents.27,34 To better identify an occluded capillary, retinas vasculature is isolated using trypsin digest and immune-stained with anti- collagen type IV to visualize basement membrane (green), anti-CD31 to visualize endothelial cells (red). (Figure 4) showed a typical example of healthy capillaries. A healthy capillary will stain red, positive for CD31, the endothelial cell marker and green, positive for type IV collagen, the marker for basement membrane resulting in yellow, colocalization of both markers. While the occluded/acellular capillary (circled) showed green only but no red staining as it contain basement membrane for endothelial cell marker. For quantification, trypsin-digested retina was stained with PASH showed significantly increased development of acellular capillaries formation (2-fold compared to control) in diabetic WT mice compared to WT controls (Figure 4). Deletion of p75NTR protected the retina from development of acellular capillaries. These results came in agreement with prior report that silencing p75NTR prevented acellular capillaries in response to proNGF over expression.17

Figure 4 Genetic deletion of p75NTR mitigated diabetes-induced acellular capillaries. (C-F): Representative images of trypsinized-retina from WT or p75NTR KO controls or diabetic mice. (G): Quantitative data showing significant increase in retinal acellular capillaries after 6-month of diabetes in WT-diabetic but not in p75 KO-diabetic animals compared to respective controls. (A-B): Representative images of a typical occluded (acellular) capillary in a trypsinized retina of diabetic animals double-labelled with anti-Collagen IV (Green) and CD31 (Red).
* P < 0.005 versus control animals. n=6-8/group.
Deletion of p75NTR suppressed diabetes-induced apoptotic markers
The p75NTR has more affinity with proform of neurotrophin including proNGF to induce neuronal cell death30,35 or vascular cell death.17 WT diabetic retina showed increased activation of the stress JNK (Figure 5) and cleaved caspase-3 (Figure 5) in retina. Deletion of p75NTR also significantly reduced diabetes-induced activation and phosphorylation of JNK and cleaved caspase-3 protein expression (Figure 5). These results are consistent with previous reports showing that activation of p75NTR/JNK/caspase-3 apoptotic pathway.17,36,38
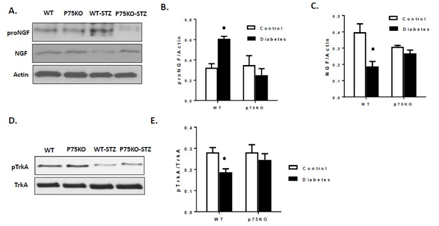
Figure 5 Genetic deletion of p75NTR suppressed diabetes-induced apoptosis in the retina. (A-C): Diabetes induced significant activation of apoptotic marker JNK, cleaved Caspase-3 compared to controls. Genetic deletion of p75NTR suppressed diabetes induced apoptotic markers in the retina compared to controls.
P < 0.05 versus control animals.
Genetic deletion of p75NTR prevented pericyte loss and restored Ephrin-B2 expression in the diabetic retina
Retinal capillary pericytes play essential role in microvascular stability and pericyte loss has been correlated with the development of DR.21,39 Ephrin-B2 is a critical protein for maintaining pericyte coverage. Ephrin-B2, a transmembrane protein with a short cytoplasmic tail, controls interactions between mural cells as well as between pericytes and the endothelial cells and key regulators of tissue morphogenesis and blood-vessel formation.40,41 To determine diabetes-induced pericyte loss in the retina, we stained the trypsin-digested retina with anti-NG2 antibody to detect pericyte (Figure 6). WT diabetic retina significantly increased pericyte loss compared to control animals. Deletion of p75NTR prevented long term diabetes-induced pericyte loss and development of acellular capillaries (Figure 6). Next, we investigated whether deletion of p75NTR altered VEGF and Ephrin-B2 expression and thereby decreasing endothelial and pericyte interaction; we measured the protein expression of VEGF and Ephrin-B2 in diabetic retina. (Figure 6) showed that diabetes tended to decrease VEGF expression in WT compared to controls, but it did not reach statistical significance.

Figure 6 Genetic deletion of p75NTR prevented pericyte loss and restored Ephrin-B2 protein expression in the diabetic retina. (A): Immunofluorescence images of NG2 staining in a trypsinized retina. (C-E): Western blot showing Diabetes induced significant decrease in Ephrin-B2 of both 37 and 60kd protein expression compared to control.
P < 0.005 versus control animals. Genetic deletion of p75NTR showed no difference in Ephrin-B2 protein expression between p75Ko control and p75ko diabetes.
* P < 0.005 versus control animals. n=6-8/group
(Figure 6) showed that diabetic significantly decreased the 37KD EFNB2 protein level and tended to decrease the 60KD EFNB2 protein level (P =0.06) compared to WT control. Interestingly, genetic deletion of p75NTR maintained Ephrin-B2 expression in diabetic retina. (Figure 6) showed that expression of both isoforms of Ephrin-B2 protein was increased in p75KO diabetic retina compared to WT-diabetic retinas. Genetic deletion of p75NTR prevented diabetes-induced pericyte loss detected at a time point of microvascular degeneration.20,21 Our results are in consistence with previous work that diabetes induced p75NTR activate NF B in endothelial cell and activated NF B act on pericyte through transferring micro particles containing microRNA-503 resulting in decreased VEGF and Ephrin-B2 expression.19 Our results are also in agreement with a recent report showing that p75NTR is mediating pericytes loss and diabetes-induced BRB breakdown.16
With alarming rate of diabetes, the prevalence of DR is on the rise. There is a great need for novel therapeutic targets that will preserve retinal neurons and vasculature. While anti-VEGF therapy have been approved, in addition to laser photocoagulation and corticosteroids. These therapeutic options are invasive and do not address the root of the problem; retinal ischemia. Our data shown that diabetes-induced p75NTR triggered retinal inflammation; IL-1β and activation of the stress kinase JNK and caspase-3-mediated apoptosis. Here, we show for the first time that upregulated expression of p75NTR is involved in disruption of neuroglia vascular unit leading to pericyte loss and development of acellular capillaries in the diabetic retina. In summary, genetic deletion of p75NTR prevented diabetes-induced increase in proNGF, mitigate inflammation and apoptosis. Deletion of p75NTR also restored NGF and TrkA activation and ephrin-B2 signaling and thereby protecting the retina during diabetes. These results suggest that targeting p75NTR may offer novel and effective therapeutic strategy to combat DR.
This work is supported by RO-1EY-022408 to Azza B. El-Remessy. This material is the result of work supported with resources and the use of facilities at the Charlie Norwood VA medical center, Augusta, GA. We would like to thank Dr. Bruce Carter (Dept. of Biochemistry, Vanderbilt University) for providing p75NTR antibody.
Authors have no conflict of interest. The contents do not represent the views of the U.S. Department of Veterans Affairs or NIH funding.

©2017 Mohamed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.