Journal of
eISSN: 2374-6947


Research Article Volume 4 Issue 4
1Banner University Medical Center, USA
10Trina Health of Oklahoma, USA
11Trina Health of Foley, USA
12Trina Health of Arizona, USA
13Trina Health of Dallas, USA
14Trina Health, USA
15NeoMark Ventures, USA
2Greater Miami Skin and Laser Center, USA
3Herbert Wertheim College of Medicine, USA
4Loma Linda University Newport Beach, USA
5Trina Health of Birmingham, USA
6Rina Health of West Los Angeles, USA
7Advanced Metabolic Care Associates, USA
8Trina Health of San Diego, USA
9Trina Health of Las Vegas, USA
Correspondence: Ford Gilbert G, Founder and CEO of Trina Health, USA
Received: June 16, 2017 | Published: July 17, 2017
Citation: Elliott J, Zaias N, Escovar S, et al. Microburst insulin infusion: results of observational studies – carbohydrate metabolism, painful diabetic neuropathy, and hospital/emergency department utilization. J Diabetes Metab Disord Control. 2017;4(4):116-121. DOI: 10.15406/jdmdc.2017.04.00118
Background: Pulsed intravenous insulin therapy (PIVIT) has shown equivocal results in studies examining metabolic control. Microburst insulin infusion mimics the periodicity and amplitude of normal pancreatic function more closely than other previous pulsatile insulin therapeutic approaches.
Methods: Data from three observational, retrospective studies are reported: carbohydrate metabolism, painful diabetic neuropathy (PDN), and hospital/emergency department utilization. All studies utilized controlled microbursts of insulin via the Bionica Microdose system in patients with type 1 and type 2diabetes. Indirect calorimetry was used to determine carbohydrate and metabolic rate measures (n=311). Painful Diabetic Neuropathy (PDN) diagnosis was assessed by the DN4 questionnaire and patients were categorized by neuropathic pain improvement (n=412). Finally, a two-year study of 1,524 diabetic patients was conducted to compare hospital admissions and emergency department visits to homologous patients.
Results: Microburst insulin infusion (MII) treatment increased oxygen consumption and energy expenditure by promoting a greater carbohydrate oxidation rate. Respiratory exchange ratio values increased significantly, reflecting a change toward carbohydrate oxidation, and resting metabolic rate increased 29%. Microburst therapy resulted in complete elimination or reduction of pain in 93% of PDN patients. Hospital admissions were reduced to 1.65/1,000/ year (versus National Hospital Discharge Survey reported rate of 46.7/1,000/year), and ED visits were reduced to 2.3/1,000/year (versus US Healthcare Statistics rate of 58.4/1,000/year).
Conclusion: Microburst insulin therapy improved underlying carbohydrate metabolism, reduced neuropathic morbidity, and reduced ED visits and hospital admissions in patients with 2 or more diabetic morbidities. Microburst insulin therapy represents a new advance in the treatment of diabetes and the complications of diabetes.
Recent research has demonstrated the burst insulin secretion of pancreatic beta cells in response to a carbohydrate load. A convincing body of evidence indicates that insulin is secreted in synchronized bursts from the pancreas into the hepatic portal vein.1-3 Multiple studies in humans and animals have described the resulting oscillatory nature of systemic levels of blood glucose and insulin.4-7 Moreover, the loss of pulsatile pancreatic activity and B-cell dysfunction may not only be initiating adverse events, but also contribute to the development of hepatic insulin resistance and progression of type 2 diabetes.8
Providing insulin in a burst fashion using a device delivering small bursts of exogenous insulin would be expected to affect insulin target tissues more effectively by more closely mimicking the secretion of insulin observed in normal individuals. In previous studies, pulsed intravenous insulin delivery has shown encouraging, yet mixed results in lowering blood glucose levels compared to equal doses of continuously infused insulin.9,10 Compared to standard therapy, burst therapy has shown promising, yet equivocal results in studies examining metabolic control, end-organ damage, and restoration of normal pulsatile pancreatic function in type 2 diabetes.11-13 Perhaps the most encouraging study on the effects of pulsed therapy on end-organ damage showed a significant preservation of renal function by pulsatile insulin infusion.14
These previous studies used earlier variations of what has been called pulsatile intravenous insulin therapy (PIVIT), which does not effectively mimic normal human insulin bursts. PIVIT, also referred to as outpatient intravenous insulin therapy (OIVIT), chronic intermittent intravenous insulin infusion (CIIIT), hepatic activation therapy (HAT), metabolic activation therapy (MAT) or the Harvard Protocol, does not replicate the periodicity and amplitude of normal pancreatic function, and was designed to deliver insulin in a series of increasing insulin peaks, producing successively increasing free insulin with limited clinical effectiveness.
In contrast, “microburst insulin infusion” (MII) via the Bionica Microdose Pump achieves the microburst of insulin causing oscillations with an amplitude of normal insulin secretion without an ever-increasing baseline of insulin – thus more closely mimicking natural burst insulin secretion.
In this report, we highlight data, which underscore the effectiveness of microburst insulin infusion in three observational, retrospective studies. These studies specifically focus on the effects of MII on metabolic rate measures, the potential attenuation and reversal of pain in patients with diabetic neuropathy, and on outcome measures as determined by hospital admissions and emergency department utilization.
All studies used MII which consisted of 3 one-hour insulin treatments, during which infused, controlled microbursts of IV insulin via the Bionica Microdose Pump, are given to match a pre-set measured oral ingestion of glucose equaling the average caloric need for that patient’s weight during the period of treatment. A rest period of up to 30 minutes is given between the one-hour microburst infusions. The treatment was calculated and delivered according to the Bionica IV infusion device Supervisor’s Guide and Trina Health protocols.15 Patients in all 3 studies continued to receive their regular regimen of hypoglycemic medication either oral or insulin.
Restoration of more normal resting metabolism of carbohydrates was assessed using the Vacumed Vista-MX2 Metabolic Measurement System, a breath-by-breath mixing chamber measurement system of the volume of carbon dioxide produced at rest. The resting carbohydrate utilization measurements were taken 3 times per treatment day. The first measurement is before initiation of MII treatment, then midway in the treatment and the third measurement at the finish of the treatment regimen. Changes in VCO2 are diagnostic, and are used to verify patient resting metabolism responses to the Trina Health protocol, and to determine the necessary treatment frequency (from once per week to once per three weeks or longer). Respiratory Quotients and VCO2 measurements were not used to guide and adjust treatment, but rather to determine the necessary frequency of treatment to maintain proper carbohydrate metabolism.
The individual treatment protocol for each patient continues until the patient’s metabolic status is maintained over a personalized period of time as shown by the ability to quickly increase carbohydrate utilization in the presence of an oral glycemic load during the first hour of treatment. The first 2 treatments are given within 1-3 days of each other, and thereafter treatment is not continuous or pre-set as to the number of days between treatments.
Carbohydrate metabolism study
Indirect calorimetry is the primary method for metabolic rate measurement and involves measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2).16 The relation between these gases and metabolic rate is defined by the respiratory exchange ratio (RER), Resting Metabolic Rate (RMR), and other measures. RER, which is a measurement of carbon dioxide produced per unit of oxygen (VCO2/VO2), varies with type of substrate (carbohydrate, fat, protein). An increased RER value is indicative of carbohydrate oxidation being favored over the oxidation of lipids for energy production. RMR is the measurement of energy required to maintain basic body functions while in a state of rest and accounts for 65% to 75% of total energy expenditure. The aim of this study was to examine the effects of MII therapy on metabolic rate measures as determined by indirect calorimetry.
Study methodology: This retrospective analysis included 311 patients treated at 12 centers, representing 7,583 individual microburst insulin treatments. Baseline and post-treatment VCO2 and VO2 metabolic measurement readings were taken for each individual treatment and comprehensive metabolic profiles were calculated. RER was determined by the respiratory exchange ratio (VCO2/VO2). Resting metabolic rate (RMR) was determined by the Weir equation.17 The Jeukendrup & Wallis equation was used to calculate carbohydrate (CHO) oxidation.18 Carbohydrate measures include % CHO oxidation of the total kcals expended, CHO kcals oxidized per liter of oxygen consumed, and CHO kcals oxidized per minute. Post-hoc analysis was performed using an orthogonal pair-wise contrasting process adjusted for within subject individual variances determined by the original repeated measures design.
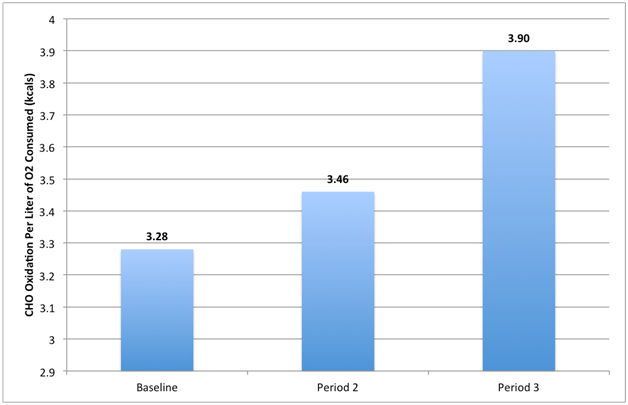
Results: Oxygen consumption significantly changed over the three measurement periods (Table 1). Oxygen consumption increased from baseline to measurement periods 2 and 3 by 1.9% (p=0.05) and 4.4% (p<0.0001), respectively. Comparing oxygen consumption between measurements 2 and 3 showed that the period 3 measurement was greater by 2.5% (p=0.015). VCO2 showed similar trends, but to a greater degree. Changes in VCO2 production comparing baseline measurement periods 2 and 3 were 2.6% (p=0.03) and 9.7% (p=0.001), respectively. Comparing measurement periods 2 and 3, VCO2 production increased 7.1% (p<0.0001). The RER values at baseline and across the other two measurement periods indicate that from baseline to period 2, the respective changes in oxygen consumption and the production of VCO2 responded similarly. RER did not significantly change initially (Baseline RER = 0.91 ±0.11; Period 2 RER =0.92 ± 0.13, p=NS). However, due to a greater relative increase in VCO2 production versus VO2 consumption in the final measurements, period 3’s RER significantly increased to both baseline and the period 2 measurement (p<0.0001). As a result, both the amount of CHO oxidized in kcals per minute and the CHO percentage of the total resting energy expenditure was significantly greater following both microburst insulin infusion treatment periods. As a result of multiple microburst insulin infusions, carbohydrate oxidation in kcals per liter of O2 increased for each measurement period (Figure 1). RMR values increased significantly from baseline to period 3 (p<0.0001) and also increased significantly from period 2 to period 3 (p=0.015). Baseline RMR values from the first visit to last visit over a period of 3 months were also compared in 27 patients in one clinic (Figure 2). Mean baseline RMR values increased from 1,475 to 1,904 (p<0.0001), an increase of 29%.
Variable |
Baseline Mean (n =311) |
95% CI |
Period 2 Mean (n=73) |
95% CI |
Period 3 Mean (n = 311) |
95% CI |
Statistical Comparisons |
VO2 (liters • min-1) |
271.7 |
263.5-279.9 |
265.8 |
249.5-282.1 |
283.8 |
275.8-283.8 |
Baseline vs Period 2: p = 0.0460 Baseline vs Period 3: p< 0.0001 Period 2 vs Period 3: p = 0.0149 |
VCO2 (liters • min-1) |
246.9 |
239.3-254.5 |
247.3 |
230.1-264.5 |
270.5 |
262.5-278.5 |
Baseline vs Period 2: p = 0.0303 Baseline vs Period 3: p< 0.0001 Period 2 vs Period 3: p <0.0001 |
RER (VCO2/VO2) |
0.91 |
0.90-0.93 |
0.93 |
0.90-0.96 |
0.96 |
0.95-0.97 |
Baseline vs Period 2: p = NS Baseline vs Period 3: p<0.0001 Period 2 vs Period 3: p<0.0001 |
CHO Oxidized (%) |
66.1 |
63.2-68.9 |
72.3 |
67.1-77.6 |
78 |
75.8-80.3 |
Baseline vs Period 2: p = 0.0450 Baseline vs Period 3: p<0.0001 Period 2 vs Period 3: p = 0.001 |
CHO Oxidized (kcals/min-1) |
0.871 |
0.827-0.916 |
0.962 |
0.867-1.057 |
1.095 |
1.051-1.140 |
Baseline vs Period 2: p = 0.0430 Baseline vs Period 3: p<0.0001 Period 2 vs Period 3: p<0.0001 |
Resting Metabolic Rate (kcals) |
1,916 |
1,861-1,972 |
1,882 |
1,774-1,990 |
2,021 |
1,966-2,077 |
Baseline vs Period 2: p = 0.3672 Baseline vs Period 3: p<0.0001 Period 2 vs Period 3: p = 0.015 |
Table 1 Metabolic Effects of Multi-Micro Insulin Burst On VO2, VCO2, and Substrate Oxidation

Study conclusion: MII treatment significantly increased oxygen consumption and energy expenditure by promoting a greater carbohydrate oxidation rate both in absolute (CHO oxidation in kcals per liter of O2 consumed and CHO oxidation in kcals per minute) and relative terms (percentage of CHO kcals expended relative the treatment groups total kcals expended per minute at rest). The increase in the respiratory exchange ratio soon after the introduction of microburst insulin therapy is to be expected, given the well-known actions of insulin on promoting glucose uptake and carbohydrate oxidation while reducing lipolysis and lipid oxidation,19 and these effects are reduced by insulin resistance, yet are still present in patients with type 2 diabetes.20 A raised RER shows that insulin is favoring carbohydrate oxidation rather than lipids for energy production. Previous investigators have reported decreases in resting metabolic rate over the short-term (2-8 days) in patients treated with insulin.21-23 It is thought that the immediate decreases in RMR may be due to the short-term underlying effects of insulin on the reduction of energy-consuming processes such as proteolysis and gluconeogenesis.24 We also report an immediate decrease in RMR in the second period following MII, consistent with previous findings. However, there was a subsequent increase in the last period, and a very significant increase of 29% in RMR values baseline-to-baseline over 3 months. These results suggest that MII has a dramatic effect on carbohydrate metabolism, overcoming the reduction of resting metabolic rate seen with conventional insulin treatments. This is of particular importance as the inability to properly metabolize carbohydrates represents a core dysfunction in diabetes. By preferentially converting energy production to carbohydrate metabolism, relative to lipids, diabetic patients avoid the consequences of elevated free fatty acids, which trigger a cascade of inflammatory processes.
Painful diabetic neuropathy study
Diabetic neuropathy is one of the most common microvascular complications of diabetes. Painful diabetic neuropathy (PDN) is reported in 16-34% of patients with diabetes.25 The symptoms of PDN can be debilitating and can cause sleep disturbances, anxiety, and interfere with physical functioning.26 The lack of normal sensation leads to injuries and eventually to amputation in many patients. PDN represents an ongoing therapeutic challenge and no single treatment exists to prevent or reverse neuropathic changes or to provide significant pain relief. Three classes of drugs are commonly used to treat diabetic neuropathy: tricyclic antidepressants, anticonvulsants, and selective serotonin-reuptake inhibitors. These compounds, however, have limited effectiveness, necessitating the need for additional pain management approaches.27 This retrospective study analyzed the effects of MII on pain associated with diabetic neuropathy.
Study methodology: The study included 412 diabetic patients (81.1% Type 2 DM, and 19.9% Type 1 DM), there were 273 males and 204 females with a mean age of 58.3 years. These patients were being treated with MII therapy as previously described. Of these 412 patents, 299 patients (72.6%) were diagnosed as having significant Painful Diabetic Neuropathy (PDN). Patients with PDN were then treated with microburst insulin therapy once per week over a period of 3 months. The diagnosis of PDN was based on clinical findings: type of pain (burning discomfort, electric shock-like sensation, aching coldness in the lower limbs); time of occurrence (most bothersome at rest and at night); and abnormal or missing sensation (such as tingling or numbness) as determined by the DN4 questionnaire.28 No neurological treatment other than MII treatment was provided, and no analgesic medications were used. Patients were then categorized based on neuropathic improvement (no improvement, significant improvement, and complete resolution), and percentages were compared (t-test, comparing percentages).
Results: After 3 months, 142 patients (47.5%) completely resolved all neuropathic symptoms, 136 patients (45.5%) reported significant improvement without complete resolution, and 21 patients (7.0%) reported no improvement (Table 2). There were significant differences (t-test) when comparing the percentages of both significantly improved and completely resolved patients to patients that were not improved (p< .0001).
No Improvement |
Significant Improvement |
N=21 (7%) |
N=136 (45.5%) |
p-value <.0001 (versus no improvement) |
Table 2 Neuropathic Improvement - One-sample t-test between percentages (n=299)
Study conclusion: Various symptomatic treatments have been proposed to manage neuropathic pain, but few have been found to be effective, with only three medications currently FDA approved for PDN. It is noteworthy that MII showed complete elimination or significant reduction of pain in 93% of PDN patients after 3 months treatment – with complete resolution of pain in 47% of PDN patients. Such significant reductions in pain may, in fact, be due to the mimicking of natural pancreatic function and restoration of carbohydrate metabolism by MII, but the exact mechanism requires further study. Given the lack of effective therapies for PDN and the encouraging findings reported in this study, additional studies on the effect of MII in PDN patients are warranted.
Hospital and emergency room utilization study
A retrospective analysis was conducted to determine if MII has a beneficial effect on patients with diabetes as determined by hospital and emergency room utilization when compared to matched National Hospital Discharge Survey and US Agency for Healthcare Statistics for homologous patients.
Methodology: This was a two-year retrospective study conducted at 14 centers involving 1,524 Type 1and Type 2 DM patients, with two or more secondary complications of diabetes (Table 3), and included patients with histories of multiple hospitalizations and emergency department visits prior to initiation of MII treatment. All study patients were treated with the MII protocol as described previously in addition to their regular hypoglycemic therapy.
Diabetic Nephropathy, stages 1 – 4 (with and without dialysis) |
Neuropathy, hands, feet, and other sites |
Cardiomyopathy, CVD |
Retinopathy, proliferative and other |
Vascular dementia, (often associated with un-healing wounds) |
Hypertension, (medication reductions) |
Un-healing wounds, (with and without osteomyelitis) |
Skin pigmentation |
Sleeplessness, Restless Leg Syndrome |
Functional energy loss |
Fibromyalgia |
Hyperlipidemia |
Hypoglycemia unawareness (T1DM) |
Black toes and feet (avoided amputations) |
Gastroparesis |
Insulin resistance |
Myasthenia gravis (MG) significant improvement |
Balance disorders |
Gait disorders, non-orthopedic |
Erectile dysfunction |
Cramping |
Depression (related) |
Depression by family/significant others |
Table 3 Comorbidities from Diabetes among study patients
Results: During the two-year study period, there were a total of 5 admissions to the hospital for treatment (Table 4). The expected hospitalization rate for patients with diabetes and two or more morbidities as determined by the National Hospital Discharge Survey (NHDS) over a two-year study period is 96 per 1000 diabetic patients (47 per 1000 diabetic patients per year).29 There were a total of 7 visits to the emergency department (ED) for the same 1,524 patients over the 2-year study period (Table 5). The expected number of emergency department visits over two years is 116 per 1,000 diabetic patients (58 per 1,000 diabetic patients per year) as determined by the US Agency for Healthcare Statistics (USAHS).30 When comparing the rates of expected hospitalization and emergency department visits versus the actual number of visits for patients treated with MII treatment, there was a significant difference between the populations (p< 0.0001).
Comparison of Two Rates (Adjusted for 2 Years) |
||
Study Visits |
NHDS Rate |
|
Numerator (e.g. number or events counted) |
5 |
94 |
Denominator (e.g. total person-years) |
1524 |
1000 |
Study incidence rate |
0.003281 |
|
95% Confidence Interval |
0.001065 to 0.007656 |
|
CDC incidence rate |
0.047 |
|
95% Confidence Interval |
0.03453 to 0.0625 |
|
Incidence rate difference |
-0.04372 |
|
95% Confidence Interval |
-0.05517 to -0.03227 |
|
P-value |
P < 0.0001 |
|
Table 4 Hospitalization Visits
Comparison of Two Rates (Adjusted For 2 Years) |
||
Study Visits |
USAHS Rate |
|
Numerator (e.g. number or events counted) |
7 |
116 |
Denominator (e.g. total person-years) |
1524 |
1000 |
Study incidence rate |
0.004593 |
|
95% Confidence Interval |
0.001847 to 0.009464 |
|
USAHS incidence rate |
0.058 |
|
95% Confidence Interval |
0.04404 to 0.07498 |
|
Incidence rate difference |
-0.05341 |
|
95% Confidence Interval |
-0.06621 to -0.04061 |
|
P-value |
P < 0.0001 |
|
Table 5 Emergency Department Visits
Study conclusion: MII reduced hospitalization in a large group of diabetics with 2 or more serious co-morbidities to 1.65/1,000/year (versus NHDS estimated rate of 46.7/1,000/year) and emergency department visits to 2.3/1,000/year (versus US for Healthcare Statistics estimated rate of 58.4/1,000/year). This represents a significant reduction in hospital admissions and emergency department visits compared to normal rates-presumably generating substantial savings for the health care system.
Pulsatile intravenous insulin therapy (PIVIT), referred to by several different names, attempted to provide insulin in a novel way, a pulsatile pattern of insulin, which met with encouraging but limited success.13,31,32 This therapy pre-dated widespread medical knowledge of how a normal pancreas secrets insulin in microbursts every 4-6 minutes.33 An alternative approach, microburst insulin infusion (MII), has been developed that is more similar to physiologic insulin release, without an ever-increasing baseline of insulin that was inherent with previous approaches (PIVIT). MII differs from PIVIT and mimics both the periodicity and amplitude of normal pancreatic insulin release more closely. This novel therapy is used as a supplement to regular hypoglycemic therapy and provides advantages in managing patients with both type 1 and type 2 diabetes and associated secondary complications. In this study, we report the results of three observational studies that utilize MII in normal, clinical settings - the first focusing on metabolic rate measures related to carbohydrate metabolism, the second on neuropathy, a common microvascular complication, and the third on hospital admission/emergency department outcome data. Significant improvements in oxygen consumption and carbon dioxide production were shown consistently over 7,583 treatments in multiple clinics, reflecting underlying improvement in carbohydrate metabolism. As far as we know, this is also the first report of an insulin treatment regimen that actually increases the resting metabolic rate (RMR) over a longer term in patients with diabetes. The increase in RMR of 29% over a period of three months is most notable. The improvements in carbohydrate metabolism by MII treatment have several implications related to systemic inflammation. The conversion of energy production to carbohydrate substrates relative to lipids, minimizes the production of free fatty acids which trigger the inflammation cascade.34 Inflammation affects insulin signaling and increases beta-cell death, and markers of inflammation such as elevated interleukin 6 (IL-6) and C-reactive protein (CRP) levels (Reference - Whitehall). Thus, MII may have considerable impact on the course of chronic inflammation in patients with diabetes. Future studies will further investigate the link between MII and inflammation, as well as the effects on markers of inflammation. Compared to traditional pharmaceutical treatment approaches for painful diabetic neuropathy, MII had notable results – with either complete elimination or significant reduction in pain in 93% of patients. These results are important as painful diabetic neuropathy (PDN) is common and is associated with significant impairment in the quality of life in patients with diabetes. Given the very few effective treatment options for patients with PDN, future studies are planned to both confirm these clinical results and investigate underlying mechanisms.
Assessment of the population health care impact of MII was demonstrated by the outcome data generated over two years, assessing hospital admissions and emergency department visits. Both these measures showed statistically significant reductions when compared to standard benchmarks, over the entire spectrum of secondary diabetes complications. Such reductions would presumably translate into substantial savings for the health care system at multiple levels. A limitation of these studies was the lack of double-blinded controls. This was deemed impractical due to the treatment duration of four hours, where glucose is given to diabetic patients with significant comorbidities. Hence, we used well-documented historical data from studies for comparative purposes. These retrospective studies we report here were typically conducted over a wide range of clinical settings and geographical locations.
In summary, conventional insulin therapy has achieved only partial success in the treatment of diabetes and prevention of chronic complications. Disappointing insulin efficacy in the treatment of diabetes may be less due to the quality of exogenous insulin than due to the possibility of physiologic “inadequacy” of its method of administration. Although insulin is secreted normally from pancreatic beta cells in a pulsatile manner, the current methods of therapeutic insulin administration are not pulsatile. Investigators have attempted to develop different methodologies to mimic normal pulsatile insulin delivery with limited success.
Microburst insulin therapy represents a new advance in the treatment of diabetes, due to its ability to closely replicate normal pancreatic function. Taken together, the rather dramatic reductions in neuropathic complications and hospital/emergency department visits, as well as the significant improvements in carbohydrate metabolism in these observational studies, may be due to the more precise mimicking of naturally pulsed insulin.
None.
Author declares that there is no conflict of interest.

©2017 Elliott, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.