Journal of
eISSN: 2374-6947


Research Article Volume 4 Issue 3
1Department of Toxicology and Narcotics, National Research Centre, Egypt
2Department of Pathology, National Research Centre, Egypt
3Department of Pharmacology, National Research Centre, Egypt
Correspondence: Omar ME Abdel-Salam, Department of Toxicology and Narcotics, National Research Centre, Tahrir St.,12311, Dokki, Cairo, Egypt
Received: October 31, 2016 | Published: April 10, 2017
Citation: Abdel-Salam OME, Shaffie NM, Sleem A. Ethamsylate protects against the carbon tetrachloride-induced liver damage in rats. J Diabetes Metab Disord Control. 2017;4(3):74-77. DOI: 10.15406/jdmdc.2017.04.00109
Ethamsylate is a synthetic haemostatic drug used for controlling capillary bleeding. In this study, the effect of ethamsylate on liver injury induced by acute carbon tetrachloride (CCl4) administration in rats was investigated. Ethamsylate (45, 90 or 180mg/kg) was given once daily orally simultaneously with CCl4 and continued for 1 week thereafter. The extent of liver damage was determined by measuring serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) as well as by liver histopathology. Ethamsylate given at the above doses conferred significant hepatic protection against the CCl4-induced hepatotoxicity. It reduced serum ALT activities by 44.8%-70.9%, AST by 46.1%-51.4%, and ALP by 30.7%-48.5%, respectively, compared to the CCl4 control group. Ethamsylate given at 90mg/kg to CCl4-treated rats resulted in the preservation of the normal architecture of the liver tissue and no lymphocytic infiltration. These data indicate a beneficial effect for ethamsylate in the CCl4 model of hepatotoxicity.
Keywords: ethamsylate, silymarin, carbon tetrachloride, liver enzymes, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase
Ethamsylate (diethylammonium 1, 4 -dihydroxy-3-benzenesulphonate) is a synthetic haemostatic drug used to reduce bleeding in conditions such as menorrhagia1 and after transurethral resection for benign enlargement of the prostate.2 The agent has also been used to reduce the risk of cerebral haemorrhage in premature infants, but controversial data have been reported.3,4 In healthy humans receiving aspirin, ethamsylate decreased the bleeding time and blood loss.5 Ethamsylate appears to ensure haemostasis by increasing platelet-leukocyte aggregates.6 The drug also exhibited other important pharmacological actions, scavenging hydroxyl radicals,7 inhibiting prostaglandin synthesis,8 decreasing inflammation9 and decreasing vascular peritoneal permeabilization due to arachidonate.7
In light of the above data and in view of the wide use of ethamsylate to control capillary bleeding, it looked pertinent to investigate the effect of the drug on the development of liver injury. The carbon tetrachloride (CCl4) model of hepatotoxicity is used for this purpose. This industrial solvent is widely used to cause acute hepatic injury with centrilobular necrosis in rodents. Moreover, its long-term administration is associated with the development of liver cirrhosis and ascites. It is thus used to investigate potential therapeutic agents.10 The development of liver injury is largely ascribed to the formation of the trichloromethyl radical, CCl3 and the ensuing oxidative cellular damage,11 although vascular,12 and inflammatory13 mechanisms are also involved.
Animals
Adult Sprague-Dawley rats of either sex, weighing 130-150 g were used in this study. Rats were fed with standard laboratory chow and water ad libitum. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Drugs and chemicals
Carbon tetrachloride (CCl4) (BDH Chemicals, UK), and ethamsylate (MINAPHARM Pharmaceuticals, Egypt) were used. The doses for rats used were based upon the human dose after conversion to that of rat according to Paget and Barnes conversion tables14 .
The CCl4 model of acute hepatic damage
The rats were divided into 5 equal groups (six rats each). Groups 1-4 received CCl4 in olive oil (1:1, vol/vol) at a dose of 2.8 ml /kg by gavage. Starting on the first day of CCl4, rats were also treated with ethamsylate (45, 90 or 180 mg/kg) once daily orally for one week. The fifth group of rats was treated with the vehicle (olive oil) at 2.8 ml/kg (no CCl4). Rats had free access to food and drinking water during the study.
Biochemical assessment
At the end of the study, blood samples were obtained from the retro-orbital venous plexus, under light ether anaesthesia. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) activities in serum were measured using commercially available kits (Biodiagnostic, Egypt).
Histopathology
After the end of the treatment period, rats were killed, the liver of different groups was removed, fixed in 10% formalin and 5 mm thick paraffin sections were stained with haematoxylin and eosin (H&E) and examined under the light microscope.
Statistical analysis
Data are expressed as means ± SE. Multiple group comparisons were performed by ANOVA followed by Duncan’s multiple range test P<0.05 was considered statistically significant.
Effect of ethamsylate on serum enzyme activities
Rats treated with CCl4 exhibited markedly increased activities of the liver enzymes ALT, AST, and ALP in plasma. Significant increase in ALT by 275.8% (71.4 ± 3.6 vs. 19.0 ± 1.0 U/L), AST by 105.8% (262.8 ± 13.0 vs. 127.7 ± 8.4 U/L) and ALP by 96.0% (519.9 ± 23.4 vs. 265.3 ± 15.6 U/L) were observed after the administration of CCl4 compared with the saline control group. Ethamsylate given at the time of CCl4 administration at doses of 45, 90 or 180 mg/kg caused dose-dependent reduction of raised ALT by 44.8%, 61.1%, and 70.9%, respectively. Meanwhile, AST decreased by 46.1%, 46.3% and 51.4% and ALP by 30.7%, 36.3%, and 48.5%, respectively (Figure 1-3).
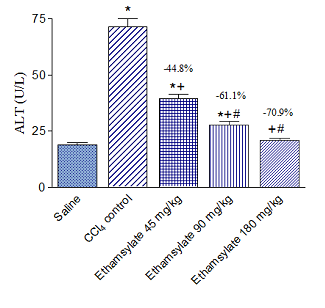
Figure 1 Effect of ethamsylate on serum ALT activity in CCl4 intoxicated rats. *p<0.05 vs. saline group. +p< 0.05 vs. CCl4 control group. #p<0.05 vs. ethamsylate 45 mg/kg treated group. The percentage change from the CCl4 control group is indicated on the figure..
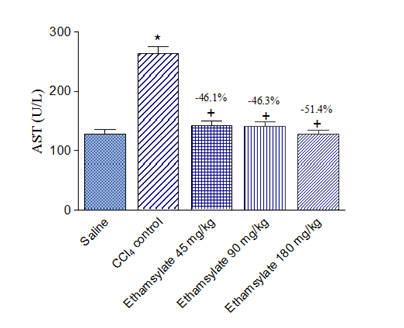
Figure 2 Effect of ethamsylate on serum AST activity in CCl4 intoxicated rats. *p<0.05 vs. saline group. +p<0.05 vs. CCl4 control group. The percentage change from the CCl4 control group is indicated on the figure.
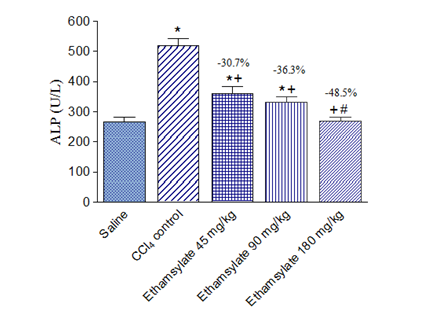
Figure 3 Effect of ethamsylate on serum ALP activity in CCl4 intoxicated rats. *<0.05 vs. saline group. +p<0.05 vs. CCl4 control group. #p<0.05 vs. ethamsylate 45 mg/kg or 90 mg/kg treated group. The percentage change from the CCl4 control group is indicated on the figure.
Effect of ethamsylate on CCl4- induced histopathological changes
Carbon tetrachloride caused marked damage to hepatic tissue in the form of severe dilatation of the portal tract vessels and central veins. There was also fibrosis that extended from a portal tract to another and dilatation of blood sinusoids (Figure 4). The administration of ethamsylate reduced the damaging effect of CCl4 in a dose- dependent manner. Minimal protection was observed with ethamsylate at 45 mg/kg as fibrosis with cellular infiltration was still observed, and hepatocytes still had pyknotic nuclei (Figure 5A & Figure 5B). Ethamsylate given at 90 mg/kg caused obvious reduction of fibrosis and cellular infiltration with preservation of liver tissue architecture (Figure 5C & Figure 5D). Ethamsylate given at 180 mg/kg afforded marked protection. The liver tissue has regained its normal structure except for very slight cellular infiltration around central veins being seen (Figure 5E & Figure 5F).
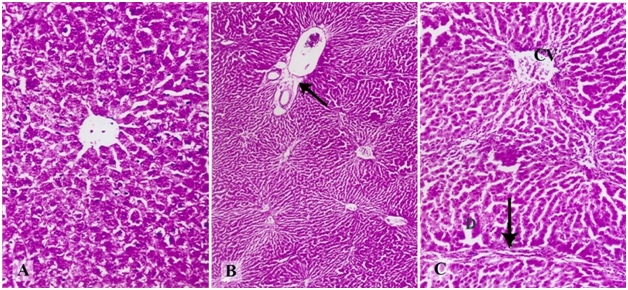
Figure 4 A photomicrograph of a section of liver tissue from a rat treated with (A) saline showing the normal hepatic architecture. (B) CCl4 showing the presence of severe dilatation of the portal tract vessels (arrow) and mild dilatation with congestion of central veins. (C) A magnified photomicrograph from the previous section showing fibrosis extending from a portal tract to another (arrow), mild dilatation with congestion in the central vein (C), and dilatation of blood sinusoids (D) (Hx & E X200, 50 & 100).
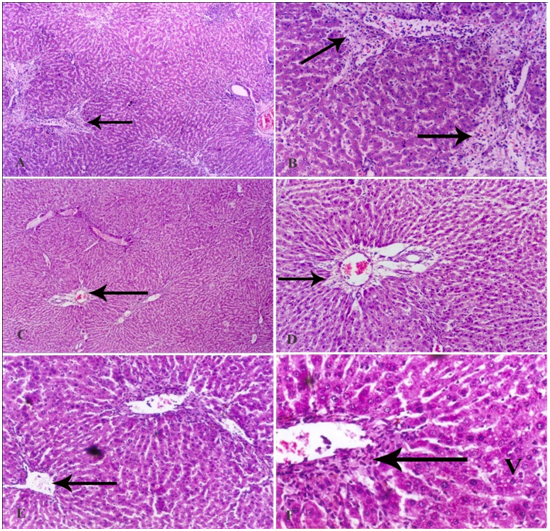
Figure 5 A photomicrograph of a section of the liver tissue from a rat treated with (A) CCl4 and 45 mg/kg ethamsylate showing dilatation and congestion of the portal tract vessels. Obvious fibrosis with lymphocytic infiltration is seen around the central veins which were also dilated and congested with blood (arrow). (B) A magnified photomicrograph for the previous section showing extensive fibrosis around a central vein with lymphocytic infiltration (arrow). The fibrous tissue extends within the parenchyma of the liver carrying lymphocytes and red blood cells. Many of the hepatocytes show small dense nuclei. Blood sinusoids are slightly dilated with increased number of Kupffer cells. (C) CCl4 + 90 mg/kg ethamsylate showing mild dilatation with congestion in the portal vein (arrow), and in some central veins. No lymphocytic infiltration is seen and the normal architecture of the liver tissue is somehow preserved. (D) A magnified photomicrograph for the previous section showing mild dilatation of the portal vein whose endothelium is still intact. Fibrosis around the portal vein (arrow) is observed. Signs of edema appear in the parenchyma of the liver in the form of dilatation of blood sinusoids. Most of the hepatocytes are still showing small dense nuclei. (E) CCl4 + 180 mg/kg ethamsylate showing preservation of the normal architecture of liver tissue and nearly normal-sized central veins (arrow). (F) A magnified photomicrograph for the previous section showing slight cellular infiltration around the central vein (arrow). Most of the hepatocytes appear normal with eosinophilic cytoplasm and large vesicular nuclei, while a few of them suffer from vacuolar degeneration (V).
The findings in the present study provide the first evidence for a protective effect for ethamsylate in the CCl4 model of hepatotoxicity. The leakage of liver enzymes into the plasma was decreased in a dose-dependent manner. The decrease in serum liver enzymes by ethamsylate clearly reflects a lower degree of liver damage. There was also histological evidence of decreased extent of liver damage, with the highest dose of ethamsylate tested restoring the normal hepatic architecture.
The CCl4-induced hepatotoxicity is due in large part to the oxidative modification of cellular biomolecules (nucleic acids, proteins, lipids). This is due to the formation of the trichloromethyl radical, CCl3 by the action of the cytochrome P450 oxygenase system. This free radical is capable of causing lipid peroxidation of the cell membrane. Moreover, in presence of molecular oxygen, CCl3 forms a highly reactive and short-lived species that is trichloromethylperoxy radical CCl3OO.11 Carbon tetrachloride thus depletes cellular antioxidant molecules like reduced glutathione.15 Ethamsylate showed antioxidant properties being particularly active against hydroxyl radicals (OH•) but not against nitric oxide radicals.7 It is likely, however, that liver injury due to CCl4 is not only caused by the oxidative insult of CCl3 and CCl3OO but also involves other factors such as vascular perturbation which add to the initial oxidative insult. In this context, it has been shown chemical sympathectomy using 6-hydroxydopamine increased hepatic blood flow and decreased liver damage caused by CCl4 in rodents.12,13
The mechanism by which ethamsylate controls bleeding is not yet explained but the drug has been shown to act by improving platelet adhesiveness and restoring capillary resistance.16 In damaged blood vessels, ethamsylate which binds to the plasma membrane of leukocytes increases the expression of the P-selectin glycoprotein ligand and promotes leukocyte-platelet aggregation.6 The above-postulated mechanisms appear to be of particular relevance to the protective action of ethamsylate against CCl4-mediated liver injury. The main physiological function of platelets is to ensure haemostasis by adhering to the sub endothelial surface of injured blood vessels forming the platelet plug.17 This anucleated blood cells store and release serotonin, a potent vasoconstrictor. Platelets also store fibrinogen, P-selectin, ATP, ADP, growth factors such as hepatocyte growth factor, vascular endothelial growth factor, platelet-derived growth factor, transforming growth factor-beta and others.18,19 Studies have indicated an important role for platelets both in the development of liver injury and also in hepatoprotection and liver regeneration.20-29 Following different forms of liver injury, platelets are recruited to the liver, adhere to endothelial lining, and cause leukocyte accumulation, sinusoidal flow disturbances20-22 and even apoptosis of sinusoidal endothelial cells.20 Adherent platelets can also release serotonin, markedly impair sinusoidal microcirculation, and decrease O2 tension.23 These actions of platelets are likely to result in exacerbation of the toxicant-induced liver damage such as that caused by CCl4. Serotonin released from platelet has also been involved in liver injury after hepatic ischemia.24,25 In other circumstances such as extensive hepatectomy, inducing thrombocytosis prevented the development of acute liver injury.26 Platelets were also proved crucial to liver regeneration and this action is mostly a serotonin-mediated one.27,28 Moreover, the release of hepatocyte growth factor from platelets is suggested to help in liver repair and sinusoidal restoration following CCl4-induced liver injury in mice.29 Platelets, therefore, might have differing effects on the development of liver injury depending on the nature of the insult and the underlying pathomechanism(s) involved. Ethamsylate increases the membrane expression of the adhesion molecule P-selectin in platelets and causes platelet adhesiveness,30 suggesting a role for the latter in the protective effects of ethamsylate observed in this study. The drug has been shown to maintain pancreatic blood flow in acute canine necrotizing pancreatitis.31 It is thus possible that the ethamsylate protects the liver via maintaining blood flow.
In summary, the present study describes for the first time a protective effect for the haemostatic drug ethamsylate in the CCl4 model of hepatotoxicity. It is suggested that ethamsylate decreases liver injury by antioxidant action as well as by virtue of an action on platelet adhesiveness or microvascular blood flow.
None.
Author declares that there is no conflict of interest.

©2017 Abdel-Salam, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.