Journal of
eISSN: 2374-6947


Mini Review Volume 3 Issue 8
1College of Life Science, Liaoning Normal University, China
2Liaoning Key Laboratories of Biotechnology and Molecular Drug Research and Development, China
3Hospital of Chinese People’s Liberation Army, China
Correspondence: Wei Zou, Liaoning Key Laboratories of Biotechnology and Molecular Drug Research and Development, China
Received: June 04, 2016 | Published: December 30, 2016
Citation: Sun J, Qu C, Wang Y, et al. Type 2 diabetes mellitus and protein-tyrosine phosphatase 1b. J Diabetes Metab Disord Control. 2016;3(8):180-183. DOI: 10.15406/jdmdc.2016.03.00096
Diabetes is one of the common metabolic diseases, mainly divided into two types, type 1 diabetes mellitus and type 2 diabetes mellitus. Insulin resistance is the main performance of type 2 diabetes mellitus which are relative to some gene mutation, genetics, obesity and so on. Protein-tyrosine phosphatase 1B (PTP1B) plays an important role as a negative regulator in insulin signaling pathways that are implicated in metabolic diseases such as obesity and type 2 diabetes. Many evidences from clinical and basical research show that the high expression of PTP1B induces insulin resistance and PTP1B is an effective target for the treatment of type 2 diabetes mellitus. In this review, we briefly introduce composition of PTP1B, and then examine the role of PTP1B in insulin signaling of type 2 diabets mellitus. Finally, we highlight the recent research progress of PTP1B inhibitors used in therapy of type 2 diabetes mellitus.
Keywords: ptp1b, type 2 diabetes mellitus, insulin signaling
Diabetes is a common metabolic disease which characterized by hyperglycemia, mainly divided into type 1 diabetes mellitus and type 2 diabetes mellitus. Insulin-dependent type 1diabetes mostly resulted from the damage of pancreatic β cells which makes the absolute lack of insulin; Type 2 diabetes, which insulin independent, the mainly initial factors are insulin resistance (IR) and the relative lack of insulin. Due to the modern diet and genetic factors, type 2 diabetes mellitus patients have increased in recent years. O’Rahilly and other experts predicted that the number of patients with type 2 diabetes mellitus will be more than 300million by 2025, mainly in developing countries such as India.1‒3 Current treatments for type 2 diabetes mellitus are mainly dependent on insulin and some oral hypoglycemic agents. Long-term insulin injections to patients with a lot of pain and inconvenience, and the effect of oral hypoglycemic agents are often not satisfactory. Therefore, researchers are now looking for more safe and effective drugs and treatments.
The process of tyrosine phosphorylation and dephosphorylation is the basic mechanism of cell growth and differentiation, and the balance of this process was maintained by protein tyrosine phosphatase (PTP) and protein tyrosine kinase (PTK).4 PTPs are superfamily of receptor-like and non- transmembrane proteins, whose members are highly specific, tighly regulated and important modulators of cellular signal initiation and termination. Protein tyrosine phosphatase 1B (PTP1B) is a key member of the family and also a negative regulator in insulin signal transduction5 and a potential targets for treatment of type 2 diabetes mellitus.6 Therefore, small-molecule PTP1B inhibitors have broad application prospects in the treatment of type 2 diabetes. Here, we briefly introduce composition of PTP1B, and then focus on the role of PTP1B in insulin signaling of type 2 diabetes mellitus and also highlight the research progress of PTP1B inhibitors used in therapy of type 2 diabetes mellitus recently.
PTP super family and PTP1B
The PTP super family can be divided into eight sub families including tyrosine-specific PTPs, DsPTPs, Cdc25, PTNE, myotubularins, PRL, LMW-PTPs and Cdc14 subfamilies, which are abundant, widely expressed as receptor-like or non-receptor in various cells. PTP1B is a representative of the intracellular PTP which was purified and identified from human placenta by Tonks for the first time in 1988.7 PTP1B is encoded by the PTPN1 gene and composed of 435 amino acid residues, with PTP family-owned conservative sequence and the molecular weight of 50kDa. It contains an N-terminal catalytic domain, two proline-rich sequences and a C-terminal hydrophobic region. The active site of PTP1B is located in the P fold of 214-221 residues, in which Cys215 and Arg221 are critical to its phosphatase catalytic sites.8 PTP1B is localized on the cytoplasmic face of the endoplasmic reticulum (ER) by means of its 35 amino acid C-terminal sequence. The catalytic center in N-terminal containing cysteine and arginine residues is towards to cytoplasm and active PTP1B released from ER after emerging hydrolytic cleavage in C-terminal.9 Price et al has identified three sensitive areas of type 2 diabetes mellitus chromosome through correlation analysis of gene markers and found that PTPN1 is located in these areas. Bento et al.10 also has published PTPN1 SNPs of high resolution map using single nucleotide polymorphism (SNP).10
PTP1B is specifically expressed in various human tissues interacted with other members of the PTPs family. In addition, PTP1B functionare regulated by several post-translational medications such as oxidation, nitrosyaltion, sulfyhydration, sumoylation, phosphorylation and proteolytic cleavage. The diverse modifications illustrated the dynamic regulation of this enzyme and its ability to modulate numerous signaling pathways likely in a cell/tissue- and stimulus-dependent manner, with high specificity and precision. PTP1B, as a potential target for type 2 diabetes and obesity, have expanded out of PTP1B gene cDNA span.11 At the same time, the molecular dynamics studies of interaction between PTP1B and its inhibitors are also going on,12 all which lay a foundation for screening specific PTP1B inhibitors.
The role of PTP1B in insulin signaling of type 2 diabetes mellitus
The pathogenesis of type 2 diabetes are associated with gene mutation, heredity, obesity and other factors, which main performance is insulin resistance. Dysfunction of pancreatic β cells is one of basic processes and characteristic of the pathogenesis. The increased prevalence of this disease highlights the urgent need to elucidate the underlying molecular mechanisms to aid in therapeutic intervention. Insulin secreted from pancreatic β cells acts as a major regulator of glucose homeostasis through a complex and integrated signaling network. Insulin receptor is a kind of transmembrane glycoprotein complex molecules, consisting of two alpha and beta subunits by three disulfide bond connection, among which the alpha subunits locate in the lateral of the cell and beta subunits are across the cell membrane. Insulin binding alpha subunits of the receptor induced phosphorylation of tyrosine residues and protein tyrosine kinase (PTK) of beta subunits, and resulted in a series of phosphorylation and dephosphorylation cascade reactions including mitogen-activated protein kinases (MAPK) and PI3K/Akt signal pathways to regulate metabolism. Whereas, when the concentration of insulin is beyond the physiological concentration (hyperinsulinemia), insulin promoted cell proliferation and developments, which may due to the combination of insulin with insulin-like growth factor 1 receptor (IGF-1R) or insulin-like growth factor 1 (IGF-1) hybrid insulin receptor, and had nothing with insulin receptor.13
Many reports indicate that PTP1B is an established metabolic regulation in mammals and a pharmacological target for type 2 diabetes. During the combination of insulin and its receptor, PTP1B could catalyze insulin receptor (IR) and insulin receptor substrates (IRS) de phosphorylation, coordinated the balance between phosphorylation and de phosphorylation of tyrosine residues, which resulted in downregulation of insulin signal transduction.14 Besides, PTP1B could de phosphorylate activated JAK2 and STAT3, and prevented leptin signal transduction (Figure 1).15 High expression of PTP1B influenced the activity of PTKs,16 which resulted in insulin failing to combine with IR, induced the insulin resistance and leptin resistance, and caused type 2 diabetes and obesity.17 Zabolotny et al.18 found that, the phosphorylation of insulin receptor tyrosine stimulated by insulin descend by 35%, the activity of PI3K decreased 40-60%, and the activity of protein kinase C (PKC) that glucose transport required also decreased in Overexpressed PTP1B mice muscle compared with the normal mice.18 Whole-body PTP1B knockout (KO) mice exhibit increased insulin sensitivity and enhanced glucose tolerance.19,20 There is relationship between occurrence of type2 diabetes and abnormality lipid metabolism. So far, amount of evidence have confirmed a causal relationship between obesity and insulin resistance in humans and animals. With weight increases, insulin sensitivity and general glucose tolerance decreased.18 Subsequent studies using tissue-specific knockout indicated that body weight, adiposity and leptin action were regulated by the neuronal PTP1B. Neuronal PTP1B deficient mice have reduced weight, adiposity, increased activity and energy consumption.21 It has also been reported that neuronal PTP1B inhibition results in decreased hypothalamic AMP-activated protein kinase (AMPK) activity in peripheral tissues and downstream gene expression changes that promote leanness and increased energy consumption. The mechanism by which PTP1B regulates adiposity and leptin sensitivity is likely to involve the coordinated regulation of AMPK in hypothalamus and peripheral tissues.22 These results demonstrated a direct role for PTP1B in down regulating the insulin and leptin functioning. These findings sparked interest in developing PTP1B inhibitors for the treatment of type 2 diabetes. Therefore, PTP1B could be used as a potential drug target23 and provide a new approach for the treatment of type 2 diabetes.
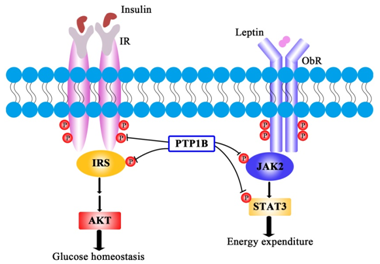
Insulin receptor mediated signal transduction, and participated in a series of cascade reactions in which protein kinase and phosphatase involved, whereas more hypoglycemic targets compounds are associated with insulin signal transduction.24 They may function by insulin receptor, CytPTK, MAPK, S6 kinases, G protein and cAMP signaling pathway.25 PTP1B, a member of PTP family, its main function is de phosphorylating the phosphorylated tyrosine residues, insulin receptor and insulin receptor substrates to negative regulate insulin signal transduction. Therefore, PTP1B inhibitors enhance insulin signaling.26,27
PTP1B inhibitors and therapy of type 2 diabetes
PTP1B is an intracellular PTP, involved in the negative regulation of the insulin as well as leptin signaling. PTP1B has emerged as a validated therapeutic target for the treatment of type 2 diabetes and related metabolic abnormalities.28 PTP1B has been inhibited experimentally using a variety of mechanisms and chemical entities. PTP1B inhibitors could potentially improve insulin resistance and normalize plasma glucose and insulin level without inducing hypoglycemia.29
Synthetic PTP1B inhibitor and therapy of type 2 diabetes: Recent years, many pharmaceutical companies have developed various PTP1B inhibitors as drug candidates for therapy of Type 2 diabetes in clinical trials, including ertiprotafib, ISIS 113715, ISIS-PTP1BRx, and trodusquemine.30 In addition, some new synthetic PTP1B inhibitors were reported such as Thiazolidinediones, Benzofuran and benzothiophene biphenyls, Vanadium complexes and Amino benzoic acid. Thiazolidinediones (TZDs) are commonly known as glitazones that share a common molecular scaffold: 2, 4-TZDs31 and can correct hyperglycemia by enhancing insulin sensitivity in target tissues and were shown to improve glycemic control by ameliorating insulin resistance in both peripheral tissues and liver in type 2 diabetic patients.32 Clinical research showed that, when metformin therapy in patients with type 2 diabetes combined with TZDs can reduce the risk of treatment failure.33 Benzofuran and benzothiophene biphenyls act at the catalytic site of the enzyme to modulate its activity. Malamas et al.34 identified two novel series of benzofuran/benzothiophene biphenyl, oxo-acetic acids and sulfonyl-salicylic acids as potent PTP1B inhibitors with good oral antihyperglycemic activity.34 Further, Murthy and Kulkarni performed 3D-QSAR study using CoMFA and CoMSIA of the above series. Comparison of 3D-QSAR contour maps with steric, electrostatic and hydrophobic properties of the PTP1B enzyme showed a high level of compatibility.35 Vanadium complexes have insulin-mimetic effects and can be used to treat complications of diabetes. Vanadate and pervanadate (the complexes of vanadate with hydrogen peroxide) are two commonly used general PTP inhibitors.36,37 Our previous study synthesized a new oxovanadium complex with 3,5-dimethyl-pyrazolyl ligand, VO(HB(3,5-Me2pz)3)(3,5-Me2pz)(SCN)(SCNH)2, showed low toxicity and significantly reduced blood glucose, blood urea nitrogen and serum creatinine levels in the diabetic mice. Additionally, p42/p44MAPK and Akt phosphorylation was markedly increased in diabetic mice and was decreased by treatment with the new oxovanadium complex.38 Amino benzoic acid compounds is a phospholipid mimetic inhibitor which directly obtained by high throughput screening method. Such compounds belong to reversible competitive inhibitor proved by enzyme kinetics and co crystallization. Zhou et al.,39 demonstrated that 2-(oxalylamino) benzoic acid inhibitors in the active site of PTP1B by means of molecular docking and CoMFA. Zhang et al.,40 indicated that CX08005 was a competitive inhibitor of PTP1B by binding to the catalytic P-loop through hydrogen bonds. In addition, various other classes of compounds have been reported to have PTP1B inhibitory potential. Including isothiazolinones,41 paracaseolide A analogs,42 phosphotyrosine mimetic,43 terpenoids44 and many more groups that show PTP1B inhibitory activity.45,46
Natural molecules as PTP1B inhibitor and therapy of type 2 diabetes: Creatures of nature synthesized a variety of novel structure and secondary metabolites during biological evolution. Secondary metabolites contain structural diversity and kind medicinal properties, and many drugs are directly or indirectly derived from natural products. What’s more, Secondary metabolites will continue to serve as new drugs. Therefore, natural products are considered as important sources for new drugs for PTP1B inhibitors.47 A wide variety of natural products have been reported with PTP1B inhibitory activity such as Morphinane alkaloid, Flavonoid, Terpenoids and so on. Morphinane alkaloid, a kind of nitrogenous alkaline organic compounds and an effective ingredients in alkaloids, are important active components in Chinese herbal medicine which widely exist in nature. It was demonstrated that berberineome, papaverine and flavonoid are all effective anti-diabetic herb through inhibite PTP1B48,49 and significantly reduce the fasting blood glucose levels.50 Flavonoid isolated from Glycyrrhiza inflata, Cyclocarya paliurus and Pongamia pinnata can also inhibiter PTP1B activity.51,52 Terpenoids as organic compounds distribute in plants, animals and marine life. They significantly inhibited the activity of PTP1B (PTP1B).53‒56 In addition, PTP1B inhibitory activity were also showed in proteoglycan, quinolone, steroids, containing nitrogen or sulfur compounds for anti-diabets.57‒59 In summary, it was found that PTP1B is the critical negative regulator in the insulin signaling pathway, and specially play an important role in the pathogenesis of type 2 diabetes mellitus. The development of small-molecule drugs for PTP1B (obtained form herb or synthetic) may be have a good future for the treatment of type 2 diabetes60
1 This work was supported by a grant from National Natural Science Foundation of China (30970353), Science and Technology Plan Projects in Liaoning Province (2015020568).
None.
Author declares that there is no conflict of interest.

©2016 Sun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.