Journal of
eISSN: 2373-4345


Research Article Volume 12 Issue 1
1Assistant Professor, Oral and Maxillofacial Surgery Department, Bangabandhu Sheikh Mujib Medical University, Bangladesh
2Professor and Chairman, Oral and Maxillofacial Surgery Department, Bangabandhu Sheikh Mujib Medical University, Bangladesh
3Department of Pathology, Faculty of Veterinary Science, Bangladesh Agricultural University, Bangladesh
4Professor and Head, Department of Surgery and Obstetrics, Faculty of Veterinary Science, Bangladesh Agricultural University, Bangladesh
5Senior Consultant, Department of Paediatrics, Cumilla Medical College, Bangladesh
Correspondence: Jachmen Sultana, Assistant Professor, PhD student, Oral and Maxillofacial Surgery Department, Bangabandhu Sheikh Mujib Medical University (BSMMU), Elysia-A2, House # 24, Road # 6, Dhanmondi - R/A, Dhaka-1205, Bangladesh
Received: December 30, 2020 | Published: January 12, 2021
Citation: Sultana DJ, Rahman PDQB, Chowdhury PDEH, et al. Clinical evaluation of growth potentiality of autogenous costochondral graft in Temporomandibular joint on New Zealand white rabbit- an experimental study. J Dent Health Oral Disord Ther. 2021;12(1):8-18. DOI: 10.15406/jdhodt.2021.12.00542
Background: Costochondral graft has been used as a substitute for a growth center in the damaged Temporomandibular joint (TMJ) in the growing children. But the relapse of the TMJ ankylosis along with facial deformity is the most common outcome after the surgery for the resorption or overgrowth of the graft. While an exploration of the human Temporomandibular joint seems to be unethical after reconstruction with an autogenous costochondral graft for study purposes, therefore we sought to determine the growth potential of the graft by clinical evaluation.
Material and methods: An experimental study was done on 96 New Zealand white male rabbits, which were divided into batches. Each batch (total 2 batches) contained 24 experimental and 24 control rabbits of known ages and species; growing (3-4) and adult (12-18) months old. TMJ arthroplasty with a costochondral graft using either 1mm or 4 mm thickness of cartilaginous cap done in both age groups. Follow-up was done regularly in batches comparing with control up to 4th, 12th, and 24th weeks of surgery to find out any relationship of behavioral change, clinical presentation, and macroscopic growth of the graft.
Results: There was a relationship among the rabbits with their behavioral change, clinical presentation, and presence of growth center in the graft. Growth was found in 60% cases, 40% was growing, and 20% in adults.
Conclusions: Costochondral graft had a 60% growth potentiality. The failure rate of 40% may be due to some unknown factors. Grafts grew in a greater number of growing rabbits than adults. Long time follow-up had a strong role in the growth of the graft. In conclusion, clinical evaluation of the rabbit model provided a fair estimation of the growth process.
Keywords: temporomandibular joint, costochondral graft, growth potentiality, clinical evaluation, New Zealand white rabbit
TMJ: Temporomandibular joint; CCG: costochondral graft; BSMMU: Bangabandhu Sheikh Mujib Medical University; icddr, b : International Centre for Diarrhoeal Disease Research, Bangladesh.
Temporomandibular joint (TMJ) ankylosis in children is common in Bangladesh because of trauma, infection, systemic diseases like typhoid fever, and unknown factors.1 But irrespective of the etiology, the resultant functional loss, and facial deformity is the principal concern. It is directly proportional to the compromised cartilaginous layer of the mandibular condyle.2–4 The condylar cartilage is accredited liable for the growth and development of TMJ, 5 though Poswillo differed in1974.6 Nevertheless, different regenerative approaches especially surgical interventions on the damaged TMJ’s cartilage surface are established but failed to induce a satisfactory result.3
Reconstruction of the TMJ with an autogenous costochondral graft (CCG) is an emerging procedure, though has been used for many years to restore joint function as well as aesthetics.4 CCG is regarded as a primary growth center with inherent potential for growth.4,5 It is thought that when a rib graft is correctly placed in the glenoid fossa, facial height and symmetry are restored, an adaptation of the condylar head occurs, that supported the demands of the functional matrix, and subsequently the mandibular growth is occurred.6 Unfortunately, they still have some disadvantages related to growth patterns and relapse because of resorption or overgrowth of the graft.5,7, 8
Understanding the growth-related trend of the graft demands further investigation. While an exploration of humans is difficult after reconstruction of TMJ, the present study intended to determine the growth potential of the autogenous costochondral graft by clinical evaluation. Considering the morbidity and ethical issues, an experimental study on the rabbit model mimicked the effect of TMJ reconstruction.
This prospective follow-up study was carried out at the Department of Oral and Maxillofacial Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, and Department of Surgery and Obstetrics, Department of Pathology, Bangladesh Agricultural University, Mymensingh-2202, during the period of 1st July’ 2018 to 31st Dec’ 2019. The objective was to determine the growth potential of the CCG in the TMJ by clinical evaluation. Following the registered protocol, an experimental study was done on 96 clinically healthy outbred New Zealand white male rabbits of known species ‘Oryctolagus cuniculus’ and ages, with average body weight (1.5–2.5kg). They were purposively collected from the sources of Animal Resources Branch, Laboratory Sciences and Services Division, icddr, b, Mohakhali, Dhaka and Dhaka University Market, Katabon, Dhaka, Bangladesh, in batches and kept in a standard environment and on a nutritious diet. There were two batches each batch contained 48 (24 experimental and 24 control) rabbits, growing 3-4 months and adult or skeletally matured 12-18 months old. TMJ arthroplasty with an autogenous CCG using either 1mm or 4 mm thickness of cartilaginous cap was done in both age groups. The rabbits were allocated into sub-groups depending on the cartilaginous cap related variables and duration of follow-up. Finally, each sub-group consisted of 4 rabbits, which were sacrificed after the end of the 4th, 12th, and 24th weeks of surgery. Follow-up was done regularly comparing with control both pre and postoperatively to find out any relationship of behavioral change, clinical presentation, and macroscopic growth of the graft. The rabbits were killed humanely following euthanasia protocol using injectable agents like 5% Thiopental Sodium in a large amount appropriate for death by the cardiac route.
Experimental animals
Female rabbits were excluded to avoid estrogen-related linear bone growth.9 Growing and skeletally matured adult rabbits included to find out any Growth Hormone-related significant growth differences.10 To identify the animal during the research identifying marker was used by the date of operation. The sampling design was purposive and the sample size was determined from the knowledge of similar studies.11, 12 The number of the experimental unit allocated to each group was kept constant by replacement allocation.
Rabbits were housed singly in conventional cages following international guidelines. There was enough space for natural behaviors and where they can safely relieve stress in the postoperative period. The room had adequate light and ventilation. There was a 12:12-hour light: dark cycle, temperature range 17°C-21°C, and relative humidity 50-55% maintained.13 The cages and feeding containers were cleaned and disinfected daily basis. Preoperative acclimatization period of 10 days strictly obeyed. The surgical procedures were done in the operation theatre of Central Veterinary Hospital, 48, Kazi Alauddin Road, Dhaka-1000, Bangladesh, and nursed in the animal house of the university (BSMMU). The carcasses were disposed of as per rules of the Central Disease Investigation Laboratory, 48, Kazi Alauddin Road, Dhaka-1000, Bangladesh by instantaneous destruction of the body in the incinerator.
Anesthesia
All the rabbits were received general anesthesia under the close supervision of an expert veterinary anesthetist. The anesthetic agents Xylazine Hydrochloride (3mg/kg) followed by Ketamine Hydrochloride (30mg/kg) after five minutes were administrated intramuscularly.14 Also, anesthesia was supplemented by Halothane inhalation (up to 3.5%) with a vaporizer and O2 (4 liters/min) incrementally through a face mask to maintain anesthesia.15 Adequate anesthesia was confirmed when the ear pinch response and the pedal withdrawal reflex were absent.16
Surgical procedure
Preparation of costochondral graft
With all the aseptic preparation after proper shaving and draping the rabbit, placed on the operating table with its shaved side up and both paired legs fixed in slightly relaxed flexion with tapes. A slightly curved incision of 3-4cm was given just below the folded elbow parallel to the level of the desired rib. Dissection continued by undermining the fascia and thin panniculus carnosus muscle after retracting latissimus dorsi muscle and care was taken to avoid the lateral thoracic artery and vein. An incision was given along the pectoralis major muscle along with the white crease line at the intersection of intercostal muscles of either 5th or 6th rib after palpation. Then a periosteal incision was given along the intended rib which was advanced towards the costochondral junction. After careful dissection, the preplanned costochondral graft was taken. The perichondrium covering the cartilage and the periosteum covering the rib a few millimeters beyond the costochondral junction were kept intact with the graft. Special attention was given to prevent the exposure of the thoracic cavity and injury to the pleural membrane. In the case of the thoracic cavity perforation, a customized chest drain was inserted to maintain negative thoracic pressure.17 Then closure was done in layers with tight closure around the chest drainage tube.
The rest of the graft’s outer surface was cleared off the leftover periosteum and was roughened for the attachment to the thick posterolateral surface of the ramus of the mandible (Figure 1A–1F).
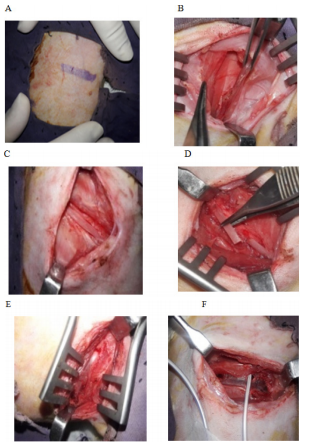
Figure 1 Surgical steps of costochondral graft harvesting technique; (A) Chest wall marking for the incision. (B) Layers of the chest wall and back muscles, the latissimus dorsi muscle retracted. (C) Rib exposed after cutting periosteum. (D) Cutting of rib. (E) Intact pleural membrane after rib elevation. (F) Chest drain fixed during pleural membrane perforation.
Reconstruction of temporomandibular joint
The condylar part was exposed by a preauricular incision of about 2.5-3 cm in length. The incision was made through the skin, subcutaneous tissue, and a thin muscle layer; which started from the level of the temporal groove and ends at the mid-level of the angle of the mandible. It was slightly convexed medially following the curvature of the posterior border of the ramus of the mandible. During dissection, at the level of the root of the auricle just behind the neck of the mandible, the facial nerve was preserved. Then a T-shaped incision was made, its horizontal portion was along with the periosteal attachment of the masseter muscle just above the zygomatic arch. The muscle retracted and part of the zygomatic arch was removed. Then the vertical portion of T continued along with the posterior attachment of the deep group of the masseter muscle in its posterior aspect; which is an avascular plane covered only by a thin layer of the temporal muscle and subcutaneous tissue. Finally, the vertical incision extended below at the level of the pedicle containing the buccal branch of the external carotid artery which was secured in this location to keep intact the vascularity. Then the dissection proceeded bluntly and the developed triangular flap containing the deep group of temporalis and masseter muscle was kept folded distally. When the condylar head was exposed, the upper part of the condylar head was excised measuring 6 mm from the condylar articular surface without disturbing the articular disc. The postero-lateral surface of the ramus of the mandible was roughened and two holes were created with a micromotor using a fissure bar for fixation purpose. Then the bony part of the graft was introduced along the channel created beneath the masseter muscle. The graft was fixed on the surface of the remaining portion of the mandible with stainless steel wire (Ortho max-26 gaze) as an on-lay and maintained a one mm gap from the articular disc. 18 Before the final tightening, the jaw movement was checked for easy mobility. Measurement was recorded from the tip of the graft to the first fixation wire for future reference. The wound was closed in layers after proper irrigation with normal saline. No drain required (Figure 2A–2F).
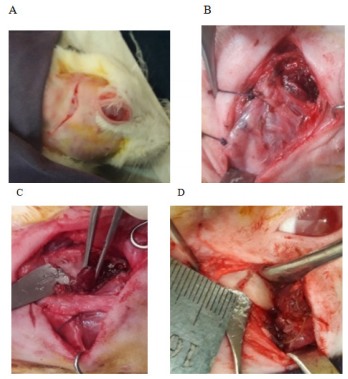
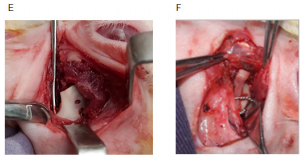
Figure 2 Reconstruction of the Temporomandibular joint with the auto transplanted costochondral graft; (A) Preauricular incision. (B) The zygomatic bone was removed. (C) Superficial and deep group of masseter muscle elevated. (D) Condylectomy cut given. (E) The hole was created for wire fixation. (F) Autogenous costochondral graft fixed keeping articular disc intact.
Immediate post-surgical care
Postsurgical care was given accordingly under the strict supervision of a veterinary specialist. Chest drain tube if required, removed as soon as the rabbit reversed, fully oriented, and moved. Analgesic and an appropriate antibiotic were provided to minimize discomfort, to prevent infection, and to promote recovery from the procedure. We provided continued and close monitoring in this period because they can deteriorate rapidly and unnoticed.
Maintenance of food and nutrition
Postoperatively rabbits were fed liquid and semisolid diet on an average of 2-4 days via nasogastric tube (6F, 8F). After that, we offered a softer, more palatable, easily digestible diet which may encourage the rabbit to eat properly. When all the methods failed to feed the rabbit gavaging done, which refers to techniques of forced feeding by forcefully restraining the rabbit. We monitored the rabbit for several days after surgery to prevent the development of post-surgical complications. Weighing the rabbit daily up to 10 days to detect postoperative health conditions. In our study we usually found there was a decrease in body weight up to 3- 5 days then they started to regain and most of them came back to their original weight on the 7th postoperative day.19 Stitches removed at 10 to 14 days postoperatively.
Behavioral assessment
After the acclimatization the rabbits were found to stay comfortably, groomed, having a good appetite, remained active and alert, they immediately reacted to any form of disturbances by erecting their ears. A healthy rabbit generally passed a copious amount of hard pellets. These behaviors were considered normal preoperatively, following surgical intervention compared with control in the postoperative period most of the gestures were either significantly reduced, or absent. Usually, we found tensed and strained posture, painful facial expression, reduced gesture of drinking, and feeding, most of the cases had signs of constipation and passed small pellets. Soft feces usually when they were not eating their caecotrophs may be due to anorexic in the postoperative period or due to antibiotics. A small fecal pellet indicates insufficient indigestible fiber, reduced food intake, or gastrointestinal hypomobility. The absence of feces indicated disruption of normal digestive function.20, 21 In this study analgesic efficacy was assessed by facial expressions as a part of behavioral markers with five action units such as- orbital tightening, cheek flattening, nose pointing and downward whisker, folded and backward position of the ear (Grimace Scale- pain scoring system on facial expressions)22 (Figure 3A–3C, Table 1A & B).
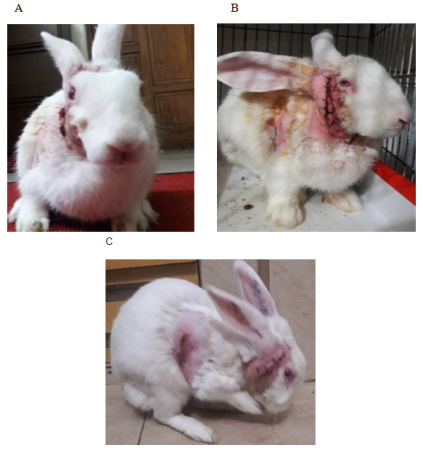
Figure 3 Postoperative changed behavior in rabbits; (A) Active and alert. (B) Gloomy painful expression. (C) Grooming rabbit.
|
Parameter |
GR |
AR |
Parameter |
GR |
AR |
Parameter |
GR |
AR |
|
Posture |
|
|
Grimace |
|
|
Grooming |
|
|
|
Day 1 |
|
|
Day 1 |
|
|
Day 1 |
|
|
|
Stay active and alert |
33.3 |
37.5 |
No Pain |
45.8 |
79.2 |
Normal |
37.5 |
45.8 |
|
Relaxed and comfortable |
20.8 |
16.7 |
Mild pain |
33.3 |
12.5 |
Disturbed |
20.8 |
25.0 |
|
No behavior at all |
25.0 |
25.0 |
Moderate pain |
12.5 |
4.2 |
Surgical site only |
16.7 |
4.2 |
|
Tensed and strained |
20.8 |
20.8 |
Severe pain |
8.3 |
4.2 |
No grooming |
25.0 |
25.0 |
|
p-value |
|
0.763 |
|
|
0.017 |
|
|
0.558 |
|
Day 3 |
|
|
Day 3 |
|
|
Day 3 |
|
|
|
Stay active and alert |
20.8 |
20.8 |
No Pain |
54.2 |
79.2 |
Normal |
29.2 |
37.5 |
|
Relaxed and comfortable |
12.5 |
16.7 |
Mild pain |
25.0 |
20.8 |
Disturbed |
25.0 |
20.8 |
|
No behavior at all |
33.3 |
37.5 |
Moderate pain |
12.5 |
0.0 |
Surgical site only |
20.8 |
33.3 |
|
Tensed and strained |
33.3 |
25.0 |
Severe pain |
8.3 |
0.0 |
No grooming |
25.0 |
8.3 |
|
p-value |
|
1.000£ |
|
|
0.066 |
|
|
0.540 |
|
Day 5 |
|
|
Day 5 |
|
|
Day 5 |
|
|
|
Stay active and alert |
37.5 |
58.3 |
No Pain |
79.2 |
100.0 |
Normal |
45.8 |
87.5 |
|
Relaxed and comfortable |
25.0 |
37.5 |
Mild pain |
20.8 |
0.0 |
Disturbed |
25.0 |
0.0 |
|
No behavior at all |
33.3 |
4.2 |
Moderate pain |
0.0 |
0.0 |
Surgical site only |
4.2 |
0.0 |
|
Tensed and strained posture |
4.2 |
0.0 |
Severe pain |
0.0 |
0.0 |
No grooming |
25.0 |
12.5 |
|
p-value |
|
0.149 |
|
|
0.018£ |
|
|
0.002 |
|
Day 7 |
|
|
Day 7 |
|
|
Day 7 |
|
|
|
Stay active and alert |
58.3 |
58.3 |
No Pain |
100.0 |
100.0 |
Normal |
100.0 |
87.5 |
|
Relaxed and comfortable |
37.5 |
41.7 |
Mild pain |
0.0 |
0.0 |
Disturbed |
0.0 |
0.0 |
|
No behavior at all |
4.2 |
0.0 |
Moderate pain |
0.0 |
0.0 |
Surgical site only |
0.0 |
0.0 |
|
|
|
|
Severe pain |
0.0 |
0.0 |
No grooming |
0.0 |
12.5 |
|
p-value |
|
1.000 |
|
|
- |
|
|
0.234£ |
Table 1A Follow-up behavior of posture, pain (on Grimace Scale) and grooming
the p-value obtained by Chi-square test by 2 x 2 table
£p-value obtained from Fisher Exact test
GR, Growing rabbit; AR, Adult rabbit
|
Parameter |
GR |
AR |
Parameter |
GR |
AR |
|
Eating behavior |
|
|
Fecal output |
|
|
|
Day 1 |
|
|
Day 1 |
|
|
|
Self eat and drink |
16.7 |
25.0 |
Normal fecal pellet |
25.0 |
33.3 |
|
Self drink |
25.0 |
20.8 |
Soft feces (caecotrophs) |
12.5 |
8.3 |
|
Refused or assisted feeding |
54.2 |
37.5 |
Small fecal pellet |
50.0 |
41.7 |
|
No feeding |
4.2 |
16.7 |
Absence of feces without distension |
12.5 |
12.5 |
|
|
|
|
Absence of feces with distension |
0.0 |
4.2 |
|
p-value |
|
0.348£ |
|
|
0.464 |
|
Day 3 |
|
|
Day 3 |
|
|
|
Self eat and drink |
8.3 |
20.8 |
Normal fecal pellet |
50.0 |
41.7 |
|
Self drink |
37.5 |
25.0 |
Soft feces (caecotrophs) |
12.5 |
4.2 |
|
Refused or assisted feeding |
54.2 |
50.0 |
Small fecal pellet |
16.7 |
25.0 |
|
No feeding |
0.0 |
4.2 |
Absence of feces without distension |
8.3 |
16.7 |
|
|
|
|
Absence of feces with distension |
12.5 |
12.5 |
|
p-value |
|
1.000£ |
|
|
0.562 |
|
Day 5 |
|
|
Day 5 |
|
|
|
Self eat and drink |
83.3 |
50.0 |
Normal fecal pellet |
83.3 |
66.7 |
|
Self drink |
8.3 |
25.0 |
Soft feces (caecotrophs) |
8.3 |
0.0 |
|
Refused or assisted feeding |
8.3 |
25.0 |
Small fecal pellet |
8.3 |
20.8 |
|
No feeding |
0.0 |
0.0 |
Absence of feces without distension |
0.0 |
8.3 |
|
|
|
- |
Absence of feces with distension |
0.0 |
4.2 |
|
p-value |
|
|
|
|
0.182 |
|
Day 7 |
|
|
Day 7 |
|
|
|
Self eat and drink |
100.0 |
100.0 |
Normal fecal pellet |
100.0 |
91.7 |
|
Self drink |
0.0 |
0.0 |
Soft feces (caecotrophs) |
0.0 |
8.3 |
|
Refused or assisted feeding |
0.0 |
0.0 |
Small fecal pellet |
0.0 |
0.0 |
|
No feeding |
0.0 |
0.0 |
Absence of feces without distension |
0.0 |
0.0 |
|
|
|
|
Absence of feces with distension |
0.0 |
0.0 |
|
p-value |
|
- |
|
|
0.149£ |
Table 1B Follow-up behavior of eating behavior and fecal output
p-value obtained by Chi-square test by 2 x 2 table
£p-value obtained from Fisher Exact test
Clinical observation
Before the surgical intervention, all rabbits’ TMJ were examined clinically, functionally, and radiologically. Following reconstruction of TMJ optimum growth of CCG was defined as excellent in order to function and facial contour, when the chin point coincided with the facial midline, occlusion remained static, arch coordination remained similar to the existing with adequate jaw movement.23 A control group containing healthy rabbits of similar ages was the standard control for the experimental group both pre and postoperatively up to the 4th, 12th, and 24th weeks of surgery. To find out any sign of mandibular growth activity, the following parameters were studied regularly: facial symmetry, state of occlusion, interincisal opening, and range of jaw movement. A modified clinical assessment checklist was introduced to determine the graft’s biological response and state of growth potentiality. The lowest score suggested similar to the existing preoperative condition. A gradual increase in score allotted for each sign of poor growth. Where poor growth was considered by facial deviation on either the same side or opposite side, malocclusion, reduced interincisal opening, and altered movement of the jaw (Figure 4A–4F & Table 2).
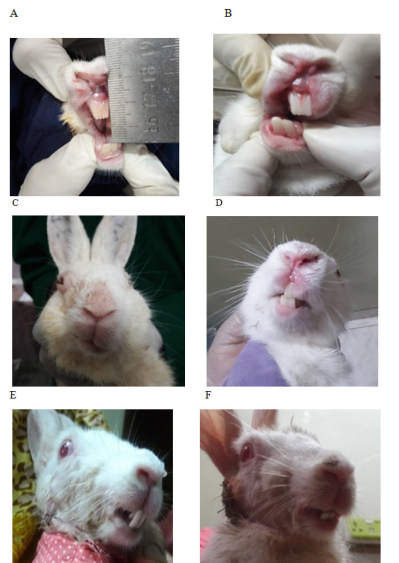
Figure 4 Clinical assessment of postoperative rabbit to determine the graft’s growth; (A) Preoperative interincisal opening. (B) Postoperative restricted and reduced oral opening. (C) Deviated face to the operated side. (D) Midline shifted to the non-operated normal side. (E) Reverse crossbite with overgrown anterior teeth. (F) Anterior open bite.
|
Parameters |
Weeks |
Growing rabbit (n=24) |
Adult rabbit (n=24) |
p-value |
||
|
No. |
% |
No. |
% |
|||
|
Facial symmetry |
||||||
|
Deviated to grafted side |
2 |
3 |
12.5 |
6 |
25 |
0.505 |
|
Deviated to opposite side |
3 |
2 |
8.3 |
1 |
4.2 |
|
|
Symmetrical (midline coincides) |
1 |
19 |
79.2 |
17 |
70.8 |
|
|
Occlusion |
||||||
|
Open bite |
3 |
0 |
0 |
2 |
8.3 |
0.001 |
|
Overgrown teeth |
4 |
1 |
4.2 |
2 |
8.3 |
|
|
Crossbite |
4 |
4 |
16.7 |
4 |
16.7 |
|
|
Premature contact |
1 |
1 |
4.2 |
1 |
4.2 |
|
|
Normal |
1 |
18 |
75 |
15 |
62.5 |
|
|
Incisal opening |
||||||
|
Severely reduced |
4 |
0 |
0 |
2 |
8.3 |
0.439 |
|
Mild reduced |
3 |
2 |
8.3 |
1 |
4.2 |
|
|
Moderate reduced |
4 |
1 |
4.2 |
2 |
8.3 |
|
|
As preoperative |
1 |
21 |
87.5 |
19 |
79.2 |
|
|
Range of jaw movement |
||||||
|
Normal (coincides with opposite side) |
1 |
17 |
70.8 |
10 |
41.7 |
0.042 |
|
Reduced excursions |
3 |
2 |
8.3 |
5 |
20.8 |
|
|
Deviated excursions |
2 |
5 |
20.8 |
7 |
29.2 |
|
|
No excursions |
4 |
0 |
0 |
2 |
8.3 |
|
Table 2 Clinical assessment of grafts growth
p-value obtained by Chi-square test by 2 x 2 table
£p-value obtained from Fisher Exact test
Occlusal bite force measurement
Though the masticatory system has a wide range of adaptive capabilities, following surgery the newly reconstructed TMJ may have functional and structural disturbances which may be reflected by reduced bite force.24 The occlusal bite force is a significant indicator of the functional state of the masticatory system.25,26 The main factors affecting the bite force in small animals are body weight, age, gender, size, breed, and the skull’s morphology.27 However, TMJ disorders, masticatory muscle atrophy, and malocclusion may also affect bite force. 25,26 In this study to measure occlusal bite force, we introduced a force-sensitive electronic device that could accurately record a wide range of force (0-100N).28 By placing the device bilaterally at the level of the first mandibular cheek tooth vertical bite force was recorded during clenching, both pre and postoperatively (Table 3).
|
Parameter
|
Occlusal bite force in Newton |
Number of growing rabbit |
Number of adult rabbit |
p-value |
||
|
Weight in Kg |
Weight in Kg |
|||||
|
>2kg |
<2kg |
>2kg |
<2kg |
|||
|
Pre-op |
12- 18 N |
14 |
10 |
17 |
7 |
0.041 |
|
Post-op -Day-3 |
2- 6 N |
8 |
4 |
11 |
6 |
0.912 |
|
Post-op-Day-5 |
6-10 N |
9 |
6 |
12 |
6 |
0.691 |
|
Post-op-Day-7 |
10-12 N |
10 |
10 |
16 |
7 |
0.190 |
|
Post-op-Day-10 |
12-18- N |
10 |
14 |
17 |
7 |
0.041 |
|
At the Day of Sacrifice |
12-18 -N |
6 |
9 |
14 |
4 |
0.027 |
Table 3 Determination of physical status or recovery sign by measurement of occlusal bite forces
p-value obtained by Chi-square test by 2 x 2 table
Macroscopic determination of growth
Consistent mandibular growth was determined macroscopically by recording the distance between the wiring point and the top of the cartilaginous cap of CCG peroperatively during initial surgery and autopsy. Macroscopic examination of the joint area was also done to identify any changes in length and width which was the presentation of graft’s growth, and its type either linear or exuberant. Poor growth was considered if there was a sign of graft resorption, overlying soft tissue fibrosis, just conversion of cartilaginous part into the bone, and ankylosis with the adjacent bony surface. Clinical types of graft’s growth:
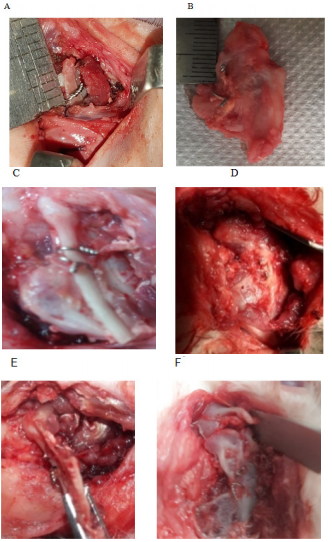
Figure 5 Macroscopic evaluation of graft’s growth; (A) Peroperative recording of graft length. (B) Measurement of graft length (exuberant type). (C) Linear type of growth (during the autopsy). (D) Severe fibrosis, (E) Graft resorption. (F) Graft grew both in length and width.
|
Parameters |
Growing rabbit (n=24) |
Adult rabbit</p> (n=24) |
p-value |
||
|
No. |
% |
No. |
% |
||
|
Graft growth |
|
|
|
|
|
|
Linear |
5 |
20.8 |
2 |
8.3 |
0.149 |
|
Exuberant |
9 |
37.5 |
7 |
29.2 |
|
|
No growth |
10 |
41.7 |
15 |
62.5 |
|
|
Graft resorption |
|
|
|
|
|
|
No resorption |
19 |
79.2 |
15 |
62.5 |
0.204 |
|
Mild |
1 |
4.2 |
1 |
4.2 |
|
|
Moderate |
0 |
0.0 |
4 |
16.7 |
|
|
Severe |
4 |
16.7 |
4 |
16.7 |
|
|
Fibrosis |
|
|
|
|
|
|
No fibrosis |
18 |
75.0 |
7 |
29.2 |
0.001£ |
|
Mild |
4 |
16.7 |
4 |
16.7 |
|
|
Moderate |
2 |
8.3 |
5 |
20.8 |
|
|
Severe |
0 |
0.0 |
8 |
33.3 |
|
|
Conversion of cartilaginous Part into bone |
3 |
12.5 |
7 |
29.2 |
0.467 |
|
Ankylosis with adjacent bone |
1 |
4.2 |
2 |
8.3 |
|
|
Macroscopic growth: |
|
|
|
|
|
|
Graft grown in width |
9 |
37.5 |
6 |
25.0 |
|
|
Graft grown in length |
2 |
12.5 |
1 |
4.2 |
|
|
Graft grown both in length & width |
3 |
12.5 |
2 |
8.3 |
|
|
No macroscopic change |
5 |
20.8 |
6 |
25.0 |
|
Table 4 Macroscopic determination of grafts’ growth
p-value obtained by Chi-square test by 2 x 2 table
£p-value obtained from Fisher Exact test
The data collection checklist and the recorded images with the help of a smartphone were used by the researcher to assess the post-surgical rabbits’ behavioral changes and clinical findings. Each observation was carried out three times, the calculated mean was recorded in the data collection sheet. An independent group of observers recorded and evaluated the follow-up observation. For standardization and reliability of data collection, there was an arrangement of both inter or intra-observer agreements.30 Rabbits weighed, occlusal bite force registered each day at 8.00 am.
Like any other autogenous graft, CCG was biologically compatible, takes less time to heal, and soon became incorporated into an adaptive mandibular condyle. There was no sign of gross infection, graft exposure, etc. Rabbits were returned to the same cage after the reversal from the anesthesia and kept in a comfortable environment under analgesics. To find out any postsurgical changed behavior, during the acclimatization period, rabbits were individually observed and recorded the baseline behavioral condition.
There was a relationship between the growing and adult rabbits in their behavioral change, clinical presentation, and macroscopic growth of the graft. The follow-up behavior in terms of posture and pain in the adult group of rabbits although showed significant improvement till day-5, the growing rabbits recovered earlier, showing signs of recovery in self-eating, grooming, and fecal output (Table 1A & 1B).
In, clinical assessment of postoperative rabbits there was no statistically significant difference found in the case of facial symmetry and incisal opening (p>0.05) though the improvement was favorable towards growing rabbits. In contrast, occlusion (p<0.001) and jaw movement (p<0.05) showed a significant difference between a growing rabbit and an adult rabbit. This finding indicated that the growing rabbit showed improvement compared to the adult rabbit (Table 2). Further, most of the rabbits those were over 2kg bodyweight had regained occlusal bite force within 7-10 days showing recovery sign (Table 3).
Growth was found in 60% cases, 40% was growing, and 20% in adults. The graft mostly grew in width than the length and of exuberant type. There was partial to complete loss of the graft in the rest of the cases (Table 4). The analysis revealed no statistically significant difference between the growing and adult groups except fibrosis (p<0.05), indicating non-fibrotic changes more pronounced in growing rabbits than adults.
This study was done to assess the clinical and functional fate of an autogenous CCG used for the reconstruction of the TMJ. After surgery, most rabbits returned to a normal state within 7 days except for a few infected cases, they were excluded from the study and replaced, for homogeneous study. The growing rabbits showed more signs of pain according to Grimace Scale. They started delayed grooming, self-eating, and drinking than adults. But they recovered at day-7 like adult rabbits. It might be due to the behavioral changes in the early postoperative period which were directly related to postsurgical pain and recovery from anesthesia.21 Most of the postsurgical rabbits with greater body mass regained occlusal bite force within 7-10 days this finding correlates with other studies.27
Facial changes consistent with graft’s growth were favorable towards the growing rabbits though a significant difference was found in terms of occlusal change and range of jaw movement with adults. Occlusal derangement like a crossbite, open bite, overgrown teeth, etc. developed in some rabbits of both age groups. Although it was managed by selective grinding of the teeth, post-surgical altered patterns of mastication followed by an over eruption of molar teeth aggravated the catastrophe over time. It was supposed to be due to graft failure or resorption, but the assessment was not always correct in every instance, because later on during autopsy it was shown that the grafts that grew were mostly undergrown. The majority of the rabbits maintained facial symmetry and normal jaw function compared with control. We recorded several data in this period, though similar data for rabbits with TMJ surgery are not available.
On macroscopic examination, the C C G matched with the mandibular condyle in shape, size, and location except for a few rabbits with a deviated face; these rabbits showed either partial to complete loss of the graft, severe fibrosis, or fusion with the adjacent bone. Though in this study there was no significant difference found between the growing and adult groups of rabbits in terms of facial symmetry and interincisal opening. In a few rabbits, the cartilage portion of the graft was converted into bone or remained unchanged morphologically. There were no signs of infection and an excellent union found between the graft and ramus of the mandible. This bone remodeling or osteogenesis process might be because of osseous metaplasia31 or previously calcified graft was used. Calcification of rib is a natural progression that should be carefully evaluated preoperatively using ultrasonography or dual-energy CT imaging 32. Due to the lack of availability of these investigations this study depended on the visual difference in the color of the costochondral junction.31
The graft's growth seen more in the growing rabbits than the adults, indicating there might be a strong influence on the growth process of growing rabbits due to hormonal and growth factors.10,29,32 In case of a few growing rabbits the graft grew mostly in width than in length and there were no sign of graft resorption. This observantion can be explained according to Timo Peltomaki et al.29 by local factors such as possible postoperative asymmetric jaw functions, and particularly uneven loading of the grafts due to changed food habbit, this might influence the structure of the graft and eventually on its growth. This scenario of asymmetrical growth found even after we maintained a one mm gap from the articular disc to prevent uneven loading on the graft. CCG with the small cartilaginous cap was straight initially so grew vertically, but the graft with the long cartilaginous cap was curved at the beginning so grew laterally then bent downwards.5 We had no experience of graft fracture, excessive hemorrhage except thoracic cavity perforation, and rupture of the pleural membrane, which was managed by a customized chest drainage system.
We faced a few drawbacks like graft resorption, conversion of a cartilaginous part into bone, ankylosis with adjacent bone, etc. Though we always kept the intact perichondrium covering the cartilage and the periosteum covering the part of the rib; which might contribute to the regeneration of the graft.32 Nevertheless, we determined the viability of C C G, its biological response, and the state of growth potentiality. It was also evident that longtime follow-up had a visible role in the graft’s growth. However, the accurate thickness of the cartilaginous cap which will restore the facial symmetry of growing children with no tendency of unexpected consequences is yet to be developed. Therefore the use of a more variable thickness of the cartilaginous cap and longtime follow-up is essential. Further, one should be aware of the differences between humans and rabbits that may affect experimental results; rabbit’s faster bone turnover, composition, and transplanted growth cartilages grow to an extent equivalent to 70-80% of their in situ growth.33, 34 Actually, no animal model can exactly duplicate the human condition, as rabbits are genetically closer to humans35 and there is a general similarity in mandibular form between rabbits and humans,36 which provided a significant research opportunity.
In the growth center concept, autogenous costochondral grafts have been used for many years for the reconstruction of damaged Temporomandibular joint (TMJ) in growing children. It is unusual to explore the joint after the reconstruction, but to date, there is no study available to assess the graft’s growth by clinical methods only. In this study, it was clinically evident that the grafts grew in a greater number of growing rabbits than adults. There was a relationship between the groups in behavioral change, clinical presentation, and macroscopic growth of the graft. The practical implementation of the results of this experimental part on growing human children will help to formulate a preformed protocol for the determination of the growth potential of the costochondral graft by clinical evaluation. Failure of the graft's growth might be because of some unknown factors or extraneous cause or maybe intrinsic and/or functional or both. In conclusion, clinical evaluation of the rabbit model provided a fair estimation of the growth process.
The authors would like to acknowledge the following institutions who provided financial support to the conduct of the research ‘Grant of Advanced Research in Education (GARE)’ of ‘Bangladesh Bureau of Education Information & Statistics (BANBEIS)’ (Grant ID: LS2018768) and the University grant commission, Dhaka University, Bangladesh, (ID-BMK/BOTI/RESAPA/COLLEGE - 05/UGCPhD (Fellow-2018). Further, the authors are grateful to and would like to thank the individuals for their efforts to make this research work fruitful were Prof. Dr. Mohammed Kamal, Prof. Dr. G H Rabbani, Prof. Dr. Ahmad Iqbal, Prof. Dr. Priya Mohan Das, Prof. Dr. Md. Mizanur Rahman.
The authors declare that they have no conflicts of interest.

©2021 Sultana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.