Journal of
eISSN: 2373-4345


Research Article Volume 8 Issue 3
1Federal University of Pelotas, Brazil
2Department of Periodontology, Karnavati School of Dentistry, India
3Department of Dentistry, Autonomous University of Hidalgo State, Mexico
Correspondence: Mohammed Irfan, Federal University of Pelotas, Rua Gonçalves Chaves, 457, Centro, CEP: 96015‐560, Pelotas, Brazil, Tel 555332256741
Received: August 17, 2017 | Published: September 25, 2017
Citation: Irfan M, Mehta T, Kumar S, et al. Comparative clinical study of coronally positioned flap with and without dehydrated amnion allograft in the treatment of gingival recession. J Dent Health Oral Disord Ther. 2017;8(3):499-505. DOI: 10.15406/jdhodt.2017.08.00281
Purpose: The aim of this study was to clinically evaluate the outcome of root coverage procedure using dehydrated amnion allograft and to compare it to the outcome of a coronally positioned flap procedure in paired marginal tissue recession defects.
Methods: Ten subjects with bilateral Miller’s class I gingival recession defects (Total 20 defects) were selected. Subjects were allocated randomly to treatment with coronally positioned flap + mnion allograft (test group) and coronally positioned flap (control group) alone. At baseline and 6 months after surgery, the following clinical parameters were recorded: Recession Depth (RD), Recession Width (RW), Probing Pocket Depth (PD), Relative Attachment Level (RAL), Width of keratinized gingiva (WKG) and Thickness of Keratinized Gingiva (TKG).
Results: The mean baseline recession was 2.9±0.87 mm and 2.5±0.90 mm for test group and control group, respectively. After 6 months, both treatments resulted in significant root coverage (P <0.01), reaching an average of 2.5 mm gain (86%) in the test group and 2.1 mm gain (84%) in the control group. The difference in recession reduction between treatments was not statistically significant. There were no statistically significant differences between the test and control groups in RD, RW, PD, CAL, and WKG. However, the mean TKG gain was 0.74 mm for the test group and 0.23 mm for the control group which was statistically significant (P<0.01).
Conclusions: It can be concluded that with or without the use of amniotic membrane coronally advanced flap provide significant root coverage in Class I gingival recessions; however, a greater keratinized tissue thickness can be obtained with the use of amniotic membrane.
Keywords: allografts, amnion, clinical study, gingival recession, membranes
A pleasing smile involves a harmonious relationship among the teeth, the gingival scaffold and the lip framework. In dental profession, aesthetics of the patient is the main factor to be focused on along with treating the disease of the patient. The primary goal of esthetic dental treatment is the restoration to a natural, healthy, and esthetic appearance from an otherwise damaged dentition.1 Today, gingival reconstruction has become a routine part of periodontal practice. The ability to cover exposed and sensitive roots and crown margins, to reconstruct lost ridges, and to enhance prosthetic reconstruction has undergone a rapid explosion.2 Numerous surgical methods and materials have been used for the treatment of gingival recession. Some of the materials used in these techniques are autogenous free gingival grafts, autogenous connective tissue grafts and allograft dermis tissue.3 Besides, biologic mediators such as enamel matrix derivative, hyaluronic acid gel and recombinant platelet derived growth factorhave been imported into surgical protocols with the intent of advancing and influencing the wound healing.4‒6 Coronally advanced flap procedure is currently seen as one of the most predictable technique available to achieve root coverage, while maintaining high aesthetics.7 Clinical results with coronally advanced flap procedure have been promising showing improved root coverage, gain in clinical attachment level (CAL) and increased width of keratinized gingiva.8 It also helps in overcoming the several disadvantages of free gingival grafts and connective tissue grafts, like the need of second surgical site, which is an additional discomfort to the patients.
Many materials have been proposed as an additional treatment to improve the clinical outcomes of basiccoronally advanced flap technique. The use of various non-absorbable membranes, like millipore filters, rubber dam, polytetrafluoroethylene membrane (PTFE) and bioabsorbable membranes like collagen membranes, polylactic acid and polyglycolic acid membranes have been advocated for guided tissue regeneration.9 Recently, allograft alternatives to autogenous tissue grafts have been proposed in the form of dermis tissue products.10 An allograft derived from human amnion tissue is an alternative option. This amnion allograft consists of a single layer of epithelium cells, thin reticular fibers (basement membrane), a thick compact layer and a fibroblast layer. The basement membrane contains collagen types III, IV, and V and bioactive factors including fibronectin and laminins. The layer acquires distinct types of laminins, with Laminin-5 being the most prevalent which simulates cellular adhesion of gingival cells and coalescing of this glycoprotein in amniotic graft may be favorable for periodontal grafting procedures.11 The self-adherent nature of the amnion allograft notably decreases the surgical time by eradicating the need for suturing and makes the procedure simple to perform relative to the techniques involving the use of autograft or allograft dermis tissue.11 In the interest of the advantages of dehydrated amnion allograft, a study was thus accomplished to compare the effectiveness of dehydrated amnion allograft with coronally positioned flap versus coronally positioned flap alone in the treatment of gingival recession. The hypothesis of the present study was stated as significant difference would be expected between experimental and control group.
Study population
This comparative clinical study was carried out in the outpatient department of Periodontics. The study protocol was explained to each potential subject and written informed consent was obtained prior to the commencement of any treatment. This study was approved by the research review board committee (KSD-234). The flow chart of the study is presented as (Figure 1). Sample size was determined by using formulae. ( Where is the critical value of the Normal distribution at ).
Inclusion criteria
The following inclusion criteria were used: Patients with age group of 18-40 years from both sexes; Presence of bilateral isolated gingival recession classified as Miller’s class I recession defect; Systemically healthy subjects and who have ability to maintain good oral hygiene; Patients willing to comply with all study related procedures and available for follow up.
Exclusion criteria
The following exclusion criteria were used: Patients having habit of smoking or chewing tobacco; Malpositioned teeth; previous surgical attempt to correct gingival recession and Pregnant or lactating women.
Procedure
A split mouth design was used in this study to minimize the bias potentially determined by difference in individual factors. Each group contained equal number of male and female participants and all patients underwent phase I therapy & oral hygiene instructions were reinforced. One month after phase I, a periodontal evaluation was performed to confirm the suitability of the sites for this study. All surgical procedures were performed by one operator. The selected sites were divided randomly (coin toss) into control and test groups. The control group sites were treated with coronally advanced flap, whereas the test group sites were treated with coronally advanced flap along with amniotic membrane application. Patients were blinded as they were not informed about the sites with amniotic membrane.
Clinical assessments
For accurate pre-operative and post-operative measurement of all the parameters, customized acrylic stents were prepared for each patient. The stents extended till the CEJ apically and at least one tooth mesially and distally. The stents were stored on the study cast to minimize the distortion. Stents were grooved in occluso-apical direction with a thin tapered bur, to allow standard UNC#15 probe to pass through it, which returned to the same position during all the successive measurements. Following clinical parameters were recorded at baseline and then postoperatively at three months and six months intervals:
Surgical procedure
After measuring all the presurgical parameters, intraoral antisepsis was performed with 0.2% chlorhexidine digluconate rinse and an iodine solution was used to carry out extraoral antisepsis. Preoperative view of gingival recession is seen in Figure 2a. After securing the local anesthesia, a horizontal incision was made at the level of the CEJ on both the sides of the tooth involved, without involving the marginal gingiva of the adjacent teeth. Incisions were given in such a way that they preserved the interdental papilla. Two vertical incisions, extending apically was given from the horizontal incisions, which were made slightly divergent to allow a broader base for better blood supply. The horizontal incisions were connected by an intrasulcular incision. A full thickness flap was raised from both horizontal incisions Figure 2b. Apical to the mucogingival junction, it was split, keeping the periosteum intact. The split thickness flap was extended into the vestibule, till the flap was pulled coronally to completely cover the gingival recession, without any tension. The adjacent interdental papillae were de-epithelialized to expose the connective tissue bed. The exposed roots were debrided with hand and ultrasonic instruments. No root biomodification was done.12 On the test site amniotic membrane (Amnio-care® Biocover Laboratories Pvt Ltd) was placed under the coronally advanced flap Figure 2c. Flap was sutured coronally with 3-0 silk sutures. Sling sutures were placed to secure flap in coronal position, and interrupted sutures, for vertical incisions Figure 2d. The periodontal dressing was given to protect the surgical area. On the control sites, the surgical procedures were identical except for the placement of the amniotic membrane.
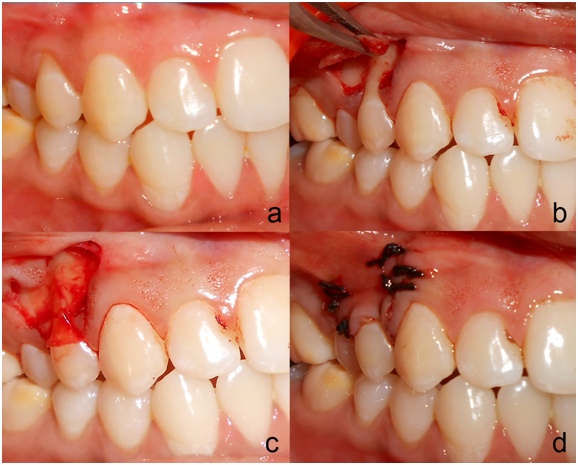
Figure 2 Preoperative view of the recession on the upper right first premolar at test side; a: Trapezoidal mucoperiosteal flap was raised up to the mucogingival junction; b: Amniotic Membrane Placed on the recipient site; c: Coronally positioned flap sutured.
Postoperative care
Suitable antibiotics and analgesics (500 mg amoxicillin, three times per day for 3 days, and 50 mg Diclofenac sodium, three times per day for 3 days) were prescribed, along with chlorhexidine digluconate rinses (0.2%) twice daily for 2 weeks. Sutures and periodontal dressings were removed 14 days post-operatively, surgical wounds were gently cleansed with 0.2% of chlorhexidine digluconate, and patients were given instructions for gentle brushing with a soft toothbrush. Each patient was instructed about proper oral hygiene measures postoperatively, examined weekly up to 1 month after surgery and at 3 and 6 months (Figure 3). At every visit, oral hygiene instructions were strengthened and the surgical areas were irrigated with normal saline. Patients were assessed clinically at the end of 3 & 6 months postoperatively. Clinical parameters (Plaque index, Modified Gingival Index, Recession depth, Recession width, Probing pocket depth, Relative attachment level, Width of keratinized gingiva, Gingival/mucosal thickness) were imitated for both control and test sites similar to previous pre surgical measurement procedures.
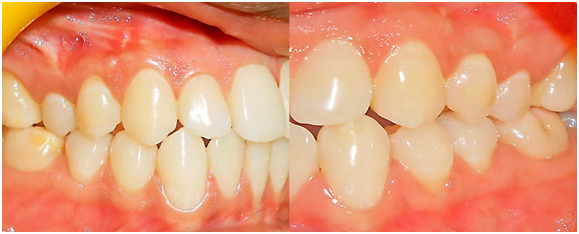
Figure 3 Postoperative view at 6 months after initial surgery at test (left) and control (right) group.
Statistics
Quantitative data were recorded as mean±standard deviation. The paired t test was used for intragroup and intergroup comparisons at 3 & 6 months. The level of significance of 0.05 was selected in all statistical observations.
Deformities of the gingiva and alveolar mucosa, which are usually referred to as mucogingival problems, are associated with a large variety of conditions that affect a large number of patients. The objective of mucogingival plastic surgery is successful coverage of exposed root surfaces, along with good esthetics and function. Gingival recession is one of the most common esthetic and functional concerns associated with periodontal tissues.13 A variety of precipitating factors have shown to be associated with recession of gingival margin: oral hygiene habits like traumatic tooth-brushing & tooth malpositioning,14 high frenum or muscle attachment,15 alveolar bone dehiscence,16 plaque & calculus17 and iatrogenic factors related to restorative and periodontal treatment procedures.18 Clinically gingival recession results in exposed root surfaces and loss of marginal tissue attachment. Along with the esthetic concerns, root surface exposure may produce dental hypersensitivity and lead to discomfort for the patient. As a result, the patient frequently avoids brushing the sensitive sites, which leads to plaque accumulation and subsequent gingival inflammation and an increased incidence of both attachments loss and root caries. To correct a lack of keratinized tissue and attain root coverage various surgical techniques have been advocated with high predictability in Miller’s Class I and II recession defects.13 These techniques include pedicle soft tissue grafts like rotational flaps and advanced flaps,7 free soft tissue grafts3 and other additive treatments like root surface modification agents, guided tissue regeneration4,5 and enamel matrix proteins. Coronally advanced technique for root coverage has been considered a predictable surgical technique for correction of gingival recession, in terms of substantial root coverage, gain in clinical attachment level and increase in width of keratinized gingiva. Various clinical studies with this technique have shown mean root coverage ranging from 45% to 95% in Miller’s Class I and II gingival recession.19,20 The advantages of CAF include ability to treat multiple areas of root exposure, with no need of involvement of adjacent teeth and high degree of success.7 The CAF technique used in the present study for gingival recession defects was the flap design described by Carlo tinti et al.21 This technique has been adapted in many studies that showed beneficial results both, alone and also along with Guided Tissue Regeneration (GTR).22 The advantage of this technique with oblique vertical incisions is that, the surgical papillae, outlined by the horizontal incisions are very wide and thus provide a larger area for anchoring the flap to the underlying vascular bed and more tissue to place the coronal sling suture. Guided tissue regeneration has been demonstrated to predictably result in the regeneration of periodontal tissues. According to Leong and Wang,23 if the objective of the treatment is not only rooting coverage, but also to obtain new attachment then procedures such as GTR-based root coverage should be considered. This GTR technique could offer several advantages over conventional surgical techniques and also the autogenous grafts, by eliminating the second surgical site for graft harvesting, less post-surgical trauma and discomfort, and increased acceptance by the patients. An additional allograft of alternative origin is derived from human amnion tissue (Amnio-care® Biocover Laboratories Pvt Ltd). Amniotic membrane (AM) is the innermost lining of the foetal membrane that is in contact with the developing fetus. This lining provides a natural barrier to secure the fetus from infections and trauma because of the paucity of a foetal immune system. It has biologic effects that are able to diminish inflammation, lessen the occurrence of adhesions and scarring, regulate angiogenesis and promote wound healing.24,25 AM also promotes epithelialization, preserve a normal epithelial phenotype and has antimicrobial properties. Amniotic membrane serves as a basement membrane which facilitates epithelial cell migration,26 reinforces adhesion of basal epithelial cells,27 promotes epithelial differentiation28 and prevents epithelial apoptosis.29 Growth factors present in the amnion tissue aid in the formation of granulation tissue by stimulating fibroblast growth and neovascularization.30 Additionally, the cells found within tissue having characteristics which are similar to that of stem cells and may magnify clinical outcomes.31 Amnion has shown a capacity to form a primitive physiologic “seal” with the host tissue and ultimately helpful in preventing bacterial contamination.32 Based on the above facts, the goal of this study was to observe the effectiveness of dehydrated amniotic membrane in conjunction with coronally advanced flap (CAF) versus coronally advanced flap alone in treatment of Miller’s class I or II gingival recession. This was a randomized controlled, single blind, split mouth clinical study. A split mouth design was used in this study to minimize the bias potentially determined by differences in individual factors. The study comprised of 10 patients (7 males and 3 females) with mean age of 37.52 years with bilaterally similar Miller’s Class I or II gingival recession defects. The sites were randomly assigned to receive either of the two treatment modalities, with the help of coin toss. Experimental site was treated with an application of amniotic membrane along with the coronally advanced flap while the control site was treated with coronally advanced flap alone. It was observed that both CAF+AM (Test) or CAF alone (Control) were capable of decreasing the amount of root exposure and obtained adequate root coverage. There was a statistically significant reduction in the recession depth in both test and control group at the end of 6 months. The mean percentage of root coverage obtained 6 months postoperatively was 86.5%±18.7% for the test group and 89.5% ± 18.32% for the control group. The percentage of root coverage is a little higher for the control group in the present study. Comparisons between the present study and the current literature are very limited. In a study conducted by Brian Gurinsky, five patient observational case series documented the use of allograft amnion in the treatment of shallow-to-moderate recession defects at three months. The average mucogingival defect size was 3.3mm (±0.84). Three months after surgery, there was an average increase of 3.2mm (±1.71) of new gingival tissue representing 97% (±0.5) defect coverage.33 In the present study for the control group, mean reduction in RD recorded was 2.2 ± 0.78 mm at 6 months, which may be comparable to the results of the study done by Cordaro et al.,34 who used the similar surgical technique for coronal advancement of flap, and found the mean RD reduction of 2.29 mm at the end of 6 months. Beneficial effects of amniotic membrane have been studied in various procedures, such as periodontal soft tissue healing,35 periodontal intrabony defects36 and in tissue engineering.37 Considering this an enhancement of root coverage was also expected in this study but the adjunction of an amniotic membrane to a CAF did not give any beneficial results in terms of percentage of root coverage at 6 months, which was 86.5±18.7 % (Test) and 89.5±18.32 % (Control). It seemed that interposing amniotic membrane, between an avascular surface and the flap was not favourable for improved root coverage and for the continuity of the marginal gingiva. The RW reduction obtained in this study is in conjunction with the amount of RD reduction achieved. The RW reduction obtained at 6 months in both the groups was statistically and clinically highly significant. (p<0.05) This finding is in conjunction with the amount of RD reduction obtained in both the groups. There was not statistically significant difference in RW reduction between the groups (p>0.05). In this study, the CAL gain obtained was similar in both the treatment groups (p>0.05). This can be explained by the fact that the amount of RD reduction obtained in both the groups was similar and there was no change in the probing depths between baseline and 6 months in both the groups. The probing depth finding of control group of the present study is also similar to the findings of probing depth in other studies.22 It has been stated that to decrease the possibility of reappearance of gingival recession and increase in the amount of keratinized tissue is needed.37 Compared with the conditions before surgical treatment, the WKG showed a statistically significant increase (p<0.05) in both the groups during the 6-month observation period. This similarity in WKG gain between the test and control groups is because of the similar amount of RD reduction obtained in the two groups. In studies evaluating the coronally repositioned flaps by Wennstorm, Pini Prato38 & Tseng SC et al.26 it has been reported that long-term increase in width of keratinized gingiva is associated with an apical shift of the mucogingival junction.38 It has also been demonstrated that extent of gingival augmentation following GTR based root coverage is positively correlated to the amount of newly formed tissue covering the previously exposed root surface. This indicates that inductive properties inherent in the newly formed tissue following GTR procedures may be involved in regulation and differentiation of overlying epithelium. For test sites, statistically significant difference was noticed at baseline and 6 months recordings of TKG. For Control sites, there was not much difference between baseline and 6 months recordings of TKG, with only 0.23 ± 0.12 mm of increase, while, for the Test site, change in TKG was 0.74 ± 0.14 mm. Thin gingival biotype increases the likelihood of gingival recession, are more prone to a deleterious effect of a hard tooth brushing technique. Thick gingival tissue is associated with more predictable surgical results, resists to trauma and promotes creeping attachment.39 In present study, the point for post-operative thickness was decided by adding 3 mm to the total gain in attachment, which allowed measuring the thickness at a constant point throughout the study period. The soft tissue augmentation in the current study may be the consequence of a gingival and periodontal ligament fibroblasts proliferation from amniotic membrane or due to a spacing effect of the amniotic membrane beneath the gingival margin. On the basis of the results achieved in the present research, it must be emphasized that the present study did not succeed in demonstrating any clinical advantage of the use of AM in combination with CAF compared to CAF alone in terms of percentage of root coverage. However, regarding the gain in thickness of keratinized gingiva, it has been shown to be advantageous over the CAF alone for long-term maintenance of the surgically established position of the soft tissue margin. Toda et al.31 studied potential of amniotic membrane/amnion-derived cells for regeneration of distinct tissues. They recorded that amnion-derived cells have multipotent differentiation ability, and these cells have attracted attention as a cell source for cell-transplantation therapy. Niknejad et al.40 studied effects of the amniotic membrane for probable use in tissue engineering. They showed that the amniotic membrane is considered a significant potential source for scaffolding material and it has other biological properties important for tissue engineering, including anti-inflammatory, anti-microbial, anti-fibrosis, anti-scarring, as well as controlled mechanical property and lesser immunogenicity.
At Baseline |
RD |
RW |
PD |
CAL |
WKG) |
TKG Mean± SD (mm) |
Test |
2.9±0.87 |
3.2±0.42 |
1.3±0.48 |
4.3±1.15 |
2.9±0.73 |
1.75±0.23 |
Control |
2.5±0.90 |
3±0.81 |
1.2±0.42 |
3.6±0.84 |
3.0±0.66 |
1.77±0.15 |
P value |
0.314 |
0.5 |
0.628 |
0.14 |
0.754 |
0.831 |
Table 1 Inter group comparison of all the parameters at baseline
Values are presented as mean±standard deviation.
RD, recession depth; RW, recession width; PD, probing pocket depth; RAL, relative attachment level; WKG, width of keratinized gingiva; TKG, thickness of keratinized gingiva
At 6 Months |
Change in RD |
Change in RW |
Change in PD |
Change in CAL |
Change in WKG |
Change in TKG |
Test |
2.5±0.84 |
2.7±0.48 |
0.3±0.48 |
2.9±1.19 |
1.8±0.63 |
0.74±0.14 |
Control |
2.2±0.78 |
2.5±0.97 |
0.1±0.31 |
2.3±0.67 |
1.3±0.48 |
0.23±0.12 |
P value |
0.424 |
0.567 |
0.288 |
0.184 |
0.062 |
<0.0001 |
Table 2 Inter group comparison of all the parameters at six months
Values are presented as mean ± standard deviation.
RD: recession depth, RW: recession width, PD: probing pocket depth, RAL: relative attachment level, WKG: Width of Keratinized Gingiva; TKG: Thickness of Keratinized Gingiva.
Parameters |
Test Group |
P- Value |
Control Group |
P- Value |
RD |
||||
Baseline |
2.9±0.87 |
<0.0001a) |
2.5±0.90 |
<0.0001a) |
6 months |
0.4± 0.51 |
0.3±0.48 |
||
RW |
||||
Baseline |
3.2±0.42 |
<0.0001a) |
3±0.81 |
<0.0001a) |
6 months |
0.5±0.52 |
0.40±0.51 |
||
PD |
||||
Baseline |
1.3±0.48 |
0.168 |
1.2±0.42 |
0.343 |
6 months |
1.0±0.0 |
1.1±0.31 |
||
RAL |
||||
Baseline |
4.3±1.15 |
<0.0001a) |
3.6±0.84 |
<0.0001a) |
6 months |
1.4±0.51 |
1.3±0.48 |
||
WKG |
||||
Baseline |
2.9±0.73 |
<0.0001a) |
3.0±0.66 |
<0.0001a) |
6 months |
4.7±0.67 |
4.3±0.67 |
||
TKG |
||||
Baseline |
1.75±0.23 |
<0.0001a) |
1.77±0.15 |
<0.0001a) |
6 months |
2.40±0.20 |
2.0±0.18 |
Table 3 Intra group comparison of all clinical parameters at baseline and 6 months
Values are presented as mean±standard deviation.
RD, recession depth; RW, recession width; PD, probing pocket depth; RAL: relative attachment level; WKG, width of keratinized gingiva; TKG, thickness of keratinized gingiva
The use of coronally advanced flap and amniotic membrane may be an effective and less invasive modality for treating Miller’s Class I gingival recession defects. In this novel technique, clinical application of amniotic membrane conserves the structural and anatomical contour of regenerated tissues. Similar root coverage can be obtained with or without the use of amniotic membrane in Class I gingival recessions; however, a greater keratinized gingival thickness can be obtained with the use of amniotic membrane. Further studies with longer follow-up and involving a larger sample size are required to evaluate the application of amniotic membrane.
None.
None.
No potential conflict of interest relevant to this article was reported.

©2017 Irfan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.