Journal of
eISSN: 2373-4345


Research Article Volume 4 Issue 1
Department of Dental Public Health, University of Dentistry and Medicine of New Jersey, US
Correspondence: Reena R Saini, Department of Dental Public Health, University of Dentistry and Medicine of New Jersey, US, Tel 4088349282
Received: June 04, 2015 | Published: January 18, 2016
Citation: Saini RR. Incidence and mortality rates of oral cancer in California from 1995-2004. J Dent Health Oral Disord Ther. 2016;4(1):10-19. DOI: 10.15406/jdhodt.2016.04.00096
Background: Oral cancer, or cancer of the oral cavity and pharynx (OCP), is an important global health concern accounting for 275,000 cases and 128,000 deaths annually. OCP cancer incidence and mortality varies markedly by sex, race/ethnicity, geographic location, and oral cancer subsite. The striking variations in the oral cancer rates underline the importance of studying the disease by these subpopulations. Epidemiological investigations describing the distribution and trends of disease between different subpopulations are crucial in developing theories to evaluate etiology, pathogenesis, and treatment so as to design possible preventative measures, screening, and early detection and implementation of relevant health policies.
Objective: To examine and analyze the age-adjusted incidence and mortality rates of OCP cancer in the state of California between the years of 1995-2004 for trends by year, gender, race, region of residence, and subsite of OCP cancer.
Methods: A total of 337,047,664 California residents between the years of 1995-2004 of ages 0 to 85+ were a part of this retrospective case series. The incidence and mortality rate data, age-adjusted per 100,000 persons, was obtained from the California Cancer Registry (CCR). Data was analyzed using SPSS.
Result: The incidence and mortality rates for both genders decreased over the ten year span of this study. Males had a 15.82 (95% CI 15.27, 16.37) incidence rate and females had a 6.57 (95% CI 6.22, 6.91) incidence rate. Males had a mortality rate of 4.21 (95% CI 3.93, 4.49) and females had a mortality rate of 1.85 (95% CI 1.70, 1.99). In terms of race, the highest incidence rate was seen in NH White males at 17.94 (95% CI 17.55, 18.34) and highest mortality rate was seen in NH Black males at 6.57 (95% CI 5.83, 7.31). For region of residence, the highest incidence rate of 12.59 (95% CI 12.10, 13.09) was seen in San Diego and the highest mortality rate of 3.21 (95% CI 2.87, 3.55) was seen in the Bay Area. In terms of oral cancer subsite, the highest incidence rate of 2.77 (95% CI 2.69, 2.86) and highest mortality rate of 0.74 (95% CI 0.71, 0.77) were both found in the tongue.
Conclusion: Males had a significantly higher incidence and mortality rate when compared to females. NH White males had the highest incidence and NH Black males had the highest mortality, whereas NH had the lowest incidence and mortality rates for each gender. San Diego had the highest age-adjusted incidence rate and Bay Area had the highest mortality. For oral cancer subsite, the tongue presented with the highest incidence and mortality rates.
The differences in incidence and mortality rates by year, gender, race, region of residence, and subsite illustrate the possible impact of varied cultural, behavioral, and biological factors in the development of disease in different ethnic subpopulations. The significant variation in subsite rates suggests the varied susceptibility to OCP cancer based on anatomical region. The difference seen in regions of residence may be attributable to demographic characteristics of the population residing within the region or environmental factors. The decreasing annual rates demonstrate the possible effect of education, prevention, and treatment on the disease. Further investigation is required to determine causality.
Keywords: Incidence; Mortality; Oral Cancer; Oropharynx; Statistical program; Hispanic
OCP, Oral Cavity and Pharynx; CCR, California Cancer Registry; SPSS, Statistical Program for Social Studies; ANOVA, Analysis of Variance
Oral cancer, the clinical problem of interest, refers to the growth of cancerous tissue in the oral cavity, which includes the mouth, the oropharynx, and the exterior of lips. Primary lesions can arise in any of the tissue types of the mouth. Most commonly oral cancer involves the tongue, but can involve the gingiva, lips, hard and soft palate, sinuses, cheek lining, salivary glands, tonsils, or oral mucosa. Although several types of oral cancer exist, squamous cell carcinomas of the mouth and lip account for approximately 95 percent of all cases of oral cancer.1
According to the Oral Cancer Foundation, in 2010, approximately 36,000 Americans will be diagnosed with oropharyngeal cancer of which only about 50 percent will survive over 5 years. The high mortality rate of oral cancer is mainly due to the tendency to discover the cancer in the late stages of development as opposed to diagnosis or treatment being difficult. Survivability of oral cancer increases dramatically if discovered in the early stages.2
Sampling
The sample size was 337,047,664 residents of California from 1995-2004. The sampling unit was residents of California of ages 0 to 85+ years. The sampling frame was residents of age 0 to 85+ who were residing in California from 1995-2004. The sampling elements have both inclusionary and exclusionary criteria.
The inclusionary criteria involved residents having the clinical features of oral cancer. The demographic features included people of all ages and gender. They must have been able to be categorized in the ethnic groups of Asian/Pacific Islander, Non-Hispanic Black, Hispanic, and Non-Hispanic White. The geographic features included residents of California.3 The temporal features span from January 1, 1995 to December 31, 2004. In terms of exclusionary criteria, temporal features exclude all data prior to January 1, 1995 and after December 31, 2004. The geographic features excluded people who are not residents of California.
In terms of sampling design, 337,047,664 residents of California from 1995-2004 were a part of the cancer study. The incidence and mortality data used were age-adjusted per 100,000 people. The sample size was determined on the basis of power analysis of a statistical test at a 0.05 level of significance.
The data was made available through the doctors, hospitals, and facilities that diagnose and treat cancer patients. California law requires cancer case information to be reported to the California Cancer Registry.4 The recruitment method was by regular oral cancer screening and by obtaining patient consent.
A comprehensive application was submitted to IRB, New York Campus of UMDNJ. The major source of data collection was the California Cancer Registry. The instrument used for data entry was a personal computer. The investigator reliability was criterion based (M.P.H. candidacy).
In this study, the variability of study variables was not addressed in terms of independent variables (i.e. tobacco use, alcohol consumption, genetics). The variability of dependent variables, incidence and mortality, was addressed.5
The validity of the study was assessed in terms of the statistical conclusion, internal validity, cause and effect, and external validity. There was a single group threat to the internal validity. History threat could be a major cause of internal validity threat because the oral cancer susceptibility of the patients could be attributed to their genetic characteristics. Co-variation of the cause and effect (i.e. if X then Y) was the criteria to meet cause and effect relationship. More than one causal factor exists for oral cancer, and therefore it posed a threat to the validity of the study. There did not exist a threat to the statistical conclusion validity because the sample size was large and homogenous. The external validity of the study was limited because the method for assigning subjects was non-random stratified sample selection.6
A pilot survey was conducted to train and calibrate the examiner. The intra-examiner and inter-examiner bias was eliminated by performing secondary data analysis and reaching an agreement between study examiners and experienced study epidemiologists.
Clinical examinations and screening for oral cancer was performed by doctors and hospitals; reports of oral cancer cases were entered into the California Cancer Registry for analysis. The age-adjusted rates per 100,000 persons for incidence and mortality of oral cancer were calculated by the California Cancer Registry. The age-adjusted rates incidence and mortality rates for year, gender, race, region, and oral cancer subsite were entered on an Excel software program and then transferred to Special Programs for Social Studies (SPSS) software, version 18. The clinical data was stored on a 640G hard drive and a 4G memory stick. The data was tested and edited for mistakes and omissions.7
Using SPSS Version 18 software packages, the significance levels between the means of assigned groups were determined and the linear models for dose-response were evaluated. Where appropriate, descriptive statistics, independent two-tailed t-tests, and analysis of variance (ANOVA) were used to determine difference among the groups.
Statistical analysis
Statistical analysis was performed with Statistical Program for Social Studies (SPSS), version 18 (SPSS Inc., Chicago, IL.) The unit of analysis was the oral cavity. The univariate descriptive statistics (mean, central tendency, dispersion, and normality) and the bivariate analysis (independent t-test) determined equivalent groups.
A power analysis was utilized to determine the sample size with one degree of freedom and based on the following components: level of statistical significance (0.05), variance (2.0), and effect size (0.20). The demographic features included gender and race.8 The geographic features included the region and state. The clinical features identified the presence of cancer and subsite. Descriptive statistics were univariate measures of mean, central tendency, dispersion and normality. Inferential statistics were derived by bivariate analysis using the independent t-test and multivariable analysis using ANOVA.
Association was the main statistical function of the statistical tests (two-tailed independent t-test and ANOVA).9 The scale of measurement of the dependent study variables (incidence and mortality) was dichotomous (Table 1, 2.1&2.2).
Incidence Rate |
95% C.I. for Mean |
|||||||
Race & Sex |
N |
Lower Range |
Upper Range |
Mean |
Std. Dev. |
Lower Bound |
Upper Bound |
Sig. |
Asian M |
10 |
9.6 |
13.5 |
11.650 |
1.3159 |
10.709 |
12.591 |
.000 |
Asian F |
10 |
5.0 |
6.7 |
5.730 |
0.5519 |
5.335 |
6.125 |
|
Black M |
10 |
15.1 |
20.6 |
17.380 |
1.5957 |
16.239 |
18.521 |
|
Black F |
10 |
4.7 |
7.0 |
6.100 |
0.8028 |
5.526 |
6.674 |
|
Hispanic M |
10 |
8.5 |
10.7 |
9.270 |
0.7875 |
8.707 |
9.833 |
|
Hispanic F |
10 |
3.1 |
4.2 |
3.770 |
0.3302 |
3.534 |
4.006 |
|
White M |
10 |
17.1 |
18.9 |
17.940 |
0.5522 |
17.545 |
18.335 |
|
White F |
10 |
6.8 |
8.1 |
7.350 |
0.4720 |
7.012 |
7.688 |
|
Total |
80 |
3.1 |
20.6 |
9.899 |
5.1039 |
8.763 |
11.035 |
|
Table 1 One-way ANOVA for Incidence Rate & Ethnicity-Sex (1995-2004)
Mortality Rate |
95% C.I. for Mean |
|
||||||
Race & Sex |
N |
Lower Range |
Upper Range |
Mean |
Std. Dev. |
Lower Bound |
Upper Bound |
Sig. |
Asian |
10 |
2.2 |
3.1 |
2.73 |
0.302 |
2.514 |
2.946 |
.000 |
NH Black |
10 |
3.5 |
4.7 |
4.08 |
0.391 |
3.8 |
4.36 |
|
Hispanic |
10 |
1.7 |
2.1 |
1.9 |
0.1333 |
1.805 |
1.995 |
|
NH White |
10 |
2.7 |
3.7 |
3.07 |
0.2946 |
2.859 |
3.281 |
|
Total |
40 |
1.7 |
4.7 |
2.945 |
0.8406 |
2.676 |
3.214 |
|
Table 2.1 One-way ANOVA for Mortality Rate & Sex (1995-2004)
Mortality Rate |
95% C.I. for Mean |
|||||||
Race & Sex |
N |
Lower Range |
Upper Range |
Mean |
Std. Dev. |
Lower Bound |
Upper Bound |
Sig. |
Asian M |
10 |
3.3 |
4.8 |
4.09 |
.5065 |
3.728 |
4.452 |
.000 |
Asian F |
10 |
1.3 |
2.0 |
1.640 |
.2633 |
1.452 |
1.828 |
|
Black M |
10 |
5.1 |
8.5 |
6.570 |
1.0371 |
5.828 |
7.312 |
|
Black F |
10 |
1.8 |
2.9 |
2.260 |
.3307 |
2.023 |
2.497 |
|
Hispanic M |
10 |
2.5 |
3.3 |
2.930 |
.2751 |
2.733 |
3.127 |
|
Hispanic F |
10 |
0.7 |
1.4 |
1.090 |
.2470 |
0.913 |
1.267 |
|
White M |
10 |
3.8 |
5.4 |
4.420 |
.4826 |
4.075 |
4.765 |
|
White F |
10 |
1.5 |
2.4 |
2.010 |
.2424 |
1.837 |
2.183 |
|
Total |
80 |
0.7 |
8.5 |
3.126 |
1.7645 |
2.734 |
3.519 |
|
Table 2.2 One-way ANOVA for Mortality Rate & Race-Sex (1995-2004)
In terms of race, Non-Hispanic Whites had the highest incidence of cancer and Hispanics had the lowest for both genders. Non-Hispanic White males had an average incidence of 17.9 and Hispanic males had an average incidence of 9.3. Non-Hispanic White males were 1.9 times more likely to develop OCP cancer as compared to Hispanic Males. Non-Hispanic White females had an average incidence of 7.4 and Hispanic females had an incidence of 3.8. Non-Hispanic White females were 1.9 times as likely to develop OCP cancer when compared with Hispanic females.
In terms of race, Non-Hispanic Blacks had the highest mortality of cancer and Hispanics had the lowest for both genders. Non-Hispanic Black males had an average mortality of 6.6 and Hispanic males had an average mortality of 2.9. Non-Hispanic Black males were 1.9 times more likely to die from OCP cancer as compared to Hispanic Males. Non-Hispanic Black females had an average mortality of 2.3 and Hispanic females had an mortality of 1.1. Non-Hispanic Black females were 2.1 times as likely to die from OCP cancer when compared with Hispanic females.
Demographic Details included the gender and race of the individual. The clinical features included the cancer subsite if the disease was present. The geographic feature was the Californian region of residence. The temporal feature was a time frame of ten years, from January 1, 1995 to December 31, 2004.
Data about the independent variables, or the cancer-causing agents was not collected. The age-adjusted incidence and mortality rate per 100,000 residents of California was examined as the dependent variable. Race, sex, region, and cancer sub site were also analyzed as modifying variables.
By year
(Graph 1) The California state average age-adjusted incidence rate for 1995-2004 was 11.17.
(Table 3) The percent rate of change of oral cavity and pharynx cancer incidence in California from 1995-2004 overall was -11.304 percent, with an average of -1.124 percent change per year.
|
Percent Rate of Change 1995-2004 |
Average Percent Rate of Change per Year |
Total Population (Incidence) |
-11.304% |
-1.256% |
Total Population (Mortality) |
-28.571% |
-3.175% |
Table 3 Incidence & Mortality of Oral Cancer, California-Percent Rate of Change 1995-2004
(Table 4) Using the Linear Regression Model, there was a significant decrease of 0.19±0.02 in the incidence rate per year and 0.07±0.02 decreases in the mortality rate per year. The incidence or mortality rate (x) can be determined using the year of diagnosis or death (y) with the formulas listed below:
Incidence: y = -0.19x + 380.42
Mortality: y = -0.07x + 143.49
(See Appendix Graph 1.1&1.2 for significant change in incidence and mortality rates by year)
|
m (slope) |
Std. Error (m) |
B (constant) |
Std. Error (B) |
P-value |
Beta |
R Square |
Incidence |
-0.19 |
0.02 |
380.42 |
37.33 |
0.00 |
-0.96 |
0.93 |
Mortality |
-0.07 |
0.02 |
143.49 |
37.18 |
0.00 |
-0.80 |
0.64 |
Table 4 Incidence & Mortality Rate Change by Year (California, 1995-2004)
By Gender:
(Graph 3, Table 5) The incidence rate for males was significantly higher in males than in females, as seen in Graph 3. Males were found to have an average incidence rate of 15.82 (95% CI 15.27, 16.37). Females had an incidence rate of 6.57 (95% CI 6.22, 6.92). Comparing the means, males were 2.4 times more likely than females to develop oral cavity and pharynx cancer.
Graph 3 Incidence counts of oral cavity & pharynx cancer by gender (age-adjusted rates per 100,000 persons).
Group Statistics |
95% C.I |
||||||
Sex |
N |
Lower Range |
Upper Range |
Mean |
Std. Dev |
Lower |
Upper |
M |
10 |
14.9 |
17 |
15.82 |
0.79 |
15.27 |
16.37 |
F |
10 |
5.9 |
7.3 |
6.57 |
0.48 |
6.22 |
6.92 |
Table 5 Independent T-Test for Oral Cancer Incidence Rates in Males & Females (1995 2005)
(Graph 4, Table 6) The mortality rate for males was significantly higher in males than in females, as seen in Graph 4. Males were found to have an average incidence rate of 4.21 (95% CI 3.93, 4.49). Females had an incidence rate of 1.85 (95% CI 1.70, 1.99). Comparing the means, males were 2.2 times more likely than females to develop oral cavity and pharynx cancer.
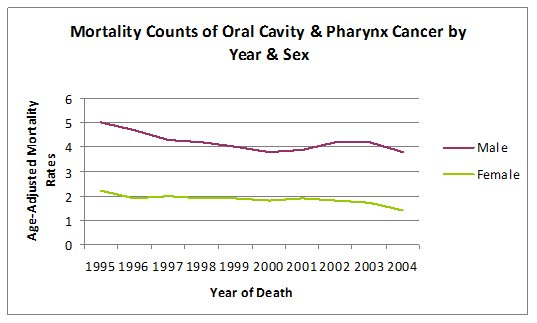
Graph 4 Mortality counts of oral cavity & pharynx cancer by gender (age-adjusted rates per 100,000 persons).
Group Statistics |
95% C.I |
||||||
Sex |
N |
Lower Range |
Upper Range |
Mean |
Std. Dev |
Lower |
Upper |
M |
10 |
3.8 |
5 |
4.21 |
0.39 |
3.93 |
4.49 |
F |
10 |
1.4 |
2.2 |
1.85 |
0.21 |
1.7 |
1.99 |
Table 6 Independent T-Test for Oral Cancer Mortality Rates in Males and Females (1995-2005)
Graph 4: Mortality Counts of Oral Cavity & Pharynx Cancer by Gender (Age-Adjusted Rates per 100,000 Persons).
(Table 7) Using the Linear Regression Model, there was a significant decrease of 0.24±0.03 in the incidence rate for males and 0.15±0.02 decreases in the incidence rate for females. The incidence rate (x) can be determined using the year of diagnosis (y) with the formulas listed below:
Male: y=-0.24x+490.85
Female: y=-0.15x+310.74
(See Appendix Graph 2.1 and 2.2 for incidence rate change by gender graphs).
|
M (slope) |
Std. Error (m) |
B (constant) |
Std. Error (B) |
P-value |
Beta |
R Square |
Male |
-0.24 |
0.03 |
490.85 |
63.21 |
0 |
-0.94 |
0.88 |
Female |
-0.15 |
0.02 |
310.74 |
34.09 |
0 |
-0.95 |
0.91 |
Table 7 Incidence Rate Change by Gender (1995-2004)
(Table 8) Using the Linear Regression Model, there was a significant decrease of 0.10±0.03 in the mortality rate for males and 0.06±0.01 decreases in the mortality rate for females. The mortality rate (x) can be determined using the year of death (y) with the formulas listed below:
Male: y=-0.10x+196.89
Female: y= 0.06x+119.40
(See Appendix Graphs 4.1 and 4.2 for mortality change by gender graphs)
|
M (slope) |
Std. Error (m) |
B (constant) |
Std. Error (B) |
P-value |
Beta |
R Square |
Male |
-0.1 |
0.03 |
196.89 |
59.42 |
0.01 |
-0.75 |
0.57 |
Female |
-0.06 |
0.01 |
119.40 |
24.6 |
0.00 |
-0.86 |
0.74 |
Table 8 Mortality Rate Change by Gender (1995-2004)
By Region
Incidence Rate |
95% C.I. for Mean |
|
||||||
Region |
N |
Lower Range |
Upper Range |
Mean |
Std. Dev. |
Lower Bound |
Upper Bound |
Sig. |
Bay Area |
10 |
10.75 |
14.02 |
12.1420 |
1.14172 |
11.3253 |
12.9587 |
.000 |
Central |
10 |
9.66 |
12.68 |
10.8090 |
.89035 |
10.1721 |
11.4459 |
|
Desert Sierra |
10 |
10.02 |
11.52 |
10.8310 |
.53590 |
10.4476 |
11.2144 |
|
Los Angeles |
10 |
9.18 |
11.08 |
10.2570 |
.66790 |
9.7792 |
10.7348 |
|
North |
10 |
11.10 |
13.69 |
12.3960 |
.85570 |
11.7839 |
13.0081 |
|
Orange County |
10 |
10.21 |
12.26 |
11.0620 |
.61142 |
10.6246 |
11.4994 |
|
Sacramento |
10 |
10.09 |
13.55 |
12.0590 |
1.06892 |
11.2943 |
12.8237 |
|
San Diego |
10 |
11.69 |
13.79 |
12.5920 |
.68621 |
12.1011 |
13.0829 |
|
Santa Clara |
10 |
9.01 |
12.22 |
10.4500 |
1.16075 |
9.6197 |
11.2803 |
|
Tri-County |
10 |
9.75 |
12.61 |
10.6700 |
1.04390 |
9.9232 |
11.4168 |
|
Total |
100 |
9.01 |
14.02 |
11.3268 |
1.19176 |
11.0903 |
11.5633 |
|
Table 9 One-way ANOVA for Incidence Rate & Region (1995-2004)
California region incidence was found to be lowest in Los Angeles at 10.3 and highest at San Diego at 12.6. San Diego had a 1.2 times higher incidence rate when compared to San Diego.
Mortality Rate |
95% C.I. for Mean |
|
||||||
Region |
N |
Lower Range |
Upper Range |
Mean |
Std. Dev. |
Lower Bound |
Upper Bound |
Sig. |
Bay Area |
10 |
2.43 |
4.25 |
3.2120 |
.47614 |
2.8714 |
3.5526 |
.000 |
Central |
10 |
2.42 |
3.58 |
2.9260 |
.38956 |
2.6473 |
3.2047 |
|
Desert Sierra |
10 |
2.78 |
3.59 |
3.1400 |
.25386 |
2.9584 |
3.3216 |
|
Los Angeles |
10 |
2.42 |
3.87 |
2.8690 |
.45337 |
2.5447 |
3.1933 |
|
North |
10 |
2.15 |
3.86 |
3.0650 |
.58694 |
2.6451 |
3.4849 |
|
Orange County |
10 |
2.11 |
3.29 |
2.6740 |
.41690 |
2.3758 |
2.9722 |
|
Sacramento |
10 |
2.74 |
3.52 |
3.1220 |
.28561 |
2.9177 |
3.3263 |
|
San Diego |
10 |
2.33 |
3.22 |
2.7220 |
.29713 |
2.5094 |
2.9346 |
|
Santa Clara |
10 |
2.00 |
3.40 |
2.6000 |
.52921 |
2.2214 |
2.9786 |
|
Tri-County |
10 |
1.38 |
2.93 |
2.2780 |
.49288 |
1.9254 |
2.6306 |
|
Total |
100 |
1.38 |
4.25 |
2.8608 |
.49757 |
2.7621 |
2.9595 |
|
Table 10 One-way ANOVA for Mortality Rate & Region (1995-2004)
The lowest mortality rate by California region was seen in Tri-County at 2.3. The highest was seen in the Bay Area at 3.2. The Bay Area had a 1.4 times higher incidence rate when compared with the Tri-County.
The age-adjusted California incidence and mortality rates from 1995-2004 were analyzed by year, sex, race, region of residence and cancer sub site.
The incidence and mortality rates for both genders decreased over the ten year span of this study. Males had a 15.82 (95% CI 15.27, 16.37) incidence rate and females had a 6.57 (95% CI 6.22, 6.91) incidence rate. Males had a mortality rate of 4.21 (95% CI 3.93, 4.49) and females had a mortality rate of 1.85 (95% CI 1.70, 1.99). In terms of race, the highest incidence rate was seen in NH White males at 17.94 (95% CI 17.55, 18.34) and highest mortality rate was seen in NH Black males at 6.57 (95% CI 5.83, 7.31). For region of residence, the highest incidence rate of 12.59 (95% CI 12.10, 13.09) was seen in San Diego and the highest mortality rate of 3.21 (95% CI 2.87, 3.55) was seen in the Bay Area. In terms of oral cancer subsite, the highest incidence rate of 2.77 (95% CI 2.69, 2.86) and highest mortality rate of 0.74 (95% CI 0.71, 0.77) were both found in the tongue.
Strengths and weaknesses
The objective for the study, to examine and analyze the incidence and mortality rates of oral cancer in the state of California between the years of 1995-2004, was met. The strengths and weaknesses of the study were determined in terms of study design, sampling plan, data collection, and statistical analysis. A weakness of the study design was in its sample groups. Given individuals of multiple ethnic backgrounds, it is an oversimplification to assign them to one of the four ethnic groups: Asian/Pacific Islander, Non-Hispanic Black, Hispanic, and Non-Hispanic White.10 Sampling was strength of the study because the California population provided for large sample size. Data collection was a weakness of the study; the nature of cancer is that as it progresses it metastasizes, and thereby making note of the primary incidence site to be difficult. Another data collection weakness was due to attrition.11 Not all residents remained in California for the entire period of the ten year study. In terms of statistical analysis, the limitations of the univariate analysis does not allow for a regression analysis; the impact of smoking, drinking, and other carcinogens and their impact on the development of oral cancer cannot be determined.12
Limitations
A limitation of this study was that the validity and reliability of the diagnosis of oral cancer is questionable because it was not based on surgical biopsies. Another limitation was that this study did not include the most recent data available on the California Cancer Registry. The Veterans Health Administration hospitals did not report cancer cases to the California Cancer Registry in 2005-2008. Case counts and incidence and mortality rates for adult males in 2005-2008 as found on the California Cancer Registry were underestimated. Therefore, the data from 2005-2008 was not included in this ten year study. Additionally, there was great variability in the incidence and mortality rates among Asians that may be attributable to their sub-ethnic group culture and behavior; the data available on the California Cancer Registry did not provide information about these subcategories [13-15]. The California Cancer Registry also did not provide information on SES, education levels, annual income, and other potential confounding variables, and so a direct relationship between these factors and incidence and mortality of OCP cancer could not be determined. Furthermore, the data available on the California Cancer Registry was aggregate data, which is susceptible to ecological fallacy.
Future research can be aimed at:
The incidence and mortality trends for oral cancer in the state of California for the years 1995-2004 were observed and analyzed. The incidence and mortality rates for both genders decreased over the ten year time span of this study in California; California followed a trend similar to the United States as a whole, which showed decreasing male and female incidence and mortality rates since the 1980s.1 Males were more likely to develop and die from oral cancer than females. The differences in race showed that Non-Hispanic Whites had the highest incidence of oral cancer. This was inconsistent with the findings of Liu et al. who found that in California, Non-Hispanic Blacks had the highest incidence rate; this may be explained by different trends found in the time period of Liu et al.’s study, which took place from 1988-2001. Non-Hispanic Blacks had the highest mortality from cancer. A higher mortality rate among Non-Hispanic Black males may be associated with SES differences or a higher tobacco and alcohol consumption rate, as seen in the study by Zygogianni et al.16 Hispanics had the lowest incidence of oral cancer. This disparity may be explained by the diversity of the Hispanic population, which arises from more than 20 different countries with various cultural, SES, and religious backgrounds.1 Region differences showed that San Diego had the highest age-adjusted incidence rate and the Bay Area had the highest mortality. These rates may be attributable to demographic characteristics or environmental factors of the given regions. For oral cancer subsite, highest incidence and mortality was found in the tongue, which was consistent with the study by Canto and Devesa.17
The differences in incidence and mortality rates by year, gender, race, region of residence, and subsite illustrate the possible impact of varied cultural, behavioral, and biological factors in the development of disease in different ethnic subpopulations. The significant variation in subsite rates suggests the varied susceptibility to OCP cancer based on anatomical region. The difference seen in regions of residence may be attributable to demographic characteristics of the population residing within the region or environmental factors.18 The decreasing annual rates of incidence and mortality of OCP cancer demonstrate the possible effect of education, prevention, and treatment on the disease. Further investigation is required to determine causality.
This study demonstrated the value of using population-based cancer registry data to study cancers with relatively low incidence and mortality to characterize rates based on sex, race, region, and subsite.19 The trends observed can be used to generate a hypothesis to determine associations and causality and to identify high-risk populations. Furthermore, it can be used to target education and early detection and prevention programs to influence lifestyle choices and reduce morbidity/mortality associated with OCP cancer.
None.
None.
Author declares that there is no conflict of interest.

©2016 Saini. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.