Journal of
eISSN: 2373-4345


Research Article Volume 3 Issue 3
1Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Future University, Egypt
2Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Mansoura University, Egypt
3Department of General Histology, Faculty of Dentistry, Future University, Egypt
Correspondence: Lobna Abdel Aziz Aly, Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Future University, Egypt, Tel 02 01001324032, Fax 02 26701496
Received: October 30, 2015 | Published: December 7, 2015
Citation: Aly LAA, Hammouda N, Ragae A. Efficacy of equine demineralized bone matrix in treating oral cyst following enucleation: a histologic and clinical study in humans. J Dent Health Oral Disord Ther. 2015;3(3):300-308. DOI: 10.15406/jdhodt.2015.03.00089
Objectives: The aim of this study is to report the effect of equine demineralized bone matrix (DBM) on the healing of oral cystic cavities following enucleation using clinical parameters.
Study design: Twelve patients aged from 20 to 40 years and suffering from cystic lesion in the jaw were included in this study. Cystic cavity augmentation with DBM was performed on 6 patients. After an average of 6 months’ healing period, a core bone was obtained and stained for histologic analysis simultaneously with implant placement.
Results: Uneventful healing and spontaneous filling of the residual cavities was obtained in all cases. All implants showed favorable Osseointegration, and final restorations were completed without failure in all cases. Histologically, new bone formation was active around grafted bone, and grafted bone was well integrated to the newly formed bone matrix. In histomorphometric analysis, vital bone volume was 25.2 ±11.9%.
Conclusion: The equine DBM is clinically useful for the increase of bone volume in cystic cavities after enucleation, because of its favorable effect of new bone formation and it is considered to be a safe, simple, reliable, acceptable, and easy handling bone grafting material.
Keywords: demineralized bone matrix, bone graft, oral cyst
DBM, demineralized bone matrix; TSE, transmissible spongiform encephalopathy; BMP, bone morphogenic protein; HEC, hydroxy ethyl cellulose; TV, tissue volume; SD, standard deviation
Although bone has the ability to repair itself following damage, extensive tissue loss due to trauma, surgical removal or disease may require the placement of a bone graft to prevent fibrous tissue in-growth and to preserve mechanical integrity.1
A bony defect in maxillofacial region presents the most challenging problem for oral and maxillofacial surgeon, therefore bone grafts are used to replace missing bone and enhance bone formation.2
Bone grafting is used to enhance healing in delayed unions, nonunions, ostoectomies, arthrodesis, and multi fragmentary fractures and to replace bony loss resulting from neoplasia or cysts.3 Autogenous bone graft is commonly used and is the standard to which allografts and graft substitutes are compared.4,5 They may provide a source of osteoprogenitor cells (osteogenesis), induce formation of osteoprogenitor cells from surrounding tissues (osteoinduction), and provide mechanical support for vascular and bone in growth (osteo conduction).6 Though autogenous bone grafts have been clinically effective, the additional surgical time required to harvest an autogenous graft, the morbidity associated with its collection, and the limited availability of autogenous bone in some patients, have encouraged the search of suitable bone graft substitutes.4,7,8 Therefore, the use of various bone graft substitutes including autografts, allografts, xenografts, polymers, ceramics and some metals have been employed to promote bone formation.8,9
Allogenic, demineralized bone matrix (DBM) has been used for several decades in human surgery for the treatment of nonunions, osteomyelitis and large defects resulting from benign tumor removal.10 The process of demineralization with hydrochloric acid destroys, but also decreases antigenic stimulation and may enhance the release of bone morphogenic protein (BMP).11 BMPs stimulate local undifferentiated mesenchymal cells to transform into osteoblasts (osteoinduction), and the collagenous framework of the DBM particles allows for migration of tissue into the site (osteoconduction). Extensive research continues to identify the different BMPs that might be osteoinductive, and these are being readied for clinical application.12,13
Demineralized bone grafts are potent osteoinductive stimulants, but have no osteoconductive properties.14 Demineralized bone matrix (DBM) is produced by carefully leaching mineral from the bone without destroying the bone morphogenetic proteins and other growth factors. DBM is available for human use in a variety of forms including fibers, flex, mouldable gels, putties, as well as an injectable version. Because DBM lacks structural properties, it is recommended only as gap filler in non weight bearing areas.15
But it is important to mention that DBM products differ concerning their biological properties due to the host environment, the methods of allograft preparation, particle size and shape and donor selection criteria. DBM functions best in a healthy tissue bed but can be expected to have little impact in an anoxic or avascular tissue bed.16
Clinical application of these bovine materials in humans is an of discussion, because of risk of transmissible spongiform encephalopathy (TSE) infection, to overcome this problem, a similar equine material, containing native BMPs was recently prepared, OSTEOPLANT ACTIVAGEN (BIOTECK products) according to the office international des epizooties, TSE has been reported in bovines, sheep, goats, cervids, farmed mink, domestic cats and exotic ungulates in zoos but not in horses. (Osteoplant Activagen, Bioteck, Italy) is a natural mixture of growth factors and is supposed to contain several BMP's and other growth factors in a collagenous matrix consisting of type I collagen.17
Advantages of DBM over other substitutes include inherent osteoinductive capacity (unlike tricalcium phosphate and hydroxy apatite) and availability in large amounts. The aim of study reported here was to is to evaluate clinically and radiographically the efficacy of equine DBM on the healing of oral cystic cavities following enucleation.
Patient selection and clinical application
Twelve patients ranging in age from 20-40 years and suffering from cystic lesion in the jaw were included in this study. The patients were selected from the outpatient clinic of oral and maxillofacial surgery department, faculty of oral and dental medicine, Future University. They were chosen at random as regard sex, site and type of the lesion. All patients were informed about the procedure and signed written informed consents. Our research has been conducted in full accordance with the World Medical Association Declaration of Helsinki, and the study has been independently reviewed and approved by an ethics committee review board at Future University.
Past and present medical and dental history was taken to assess the general condition of the patient. Through intraoral and extraoral examinations, the oral and dental condition of the patient was evaluated. All patients involved in this study are free from any debilitating condition that may interfere with bone regeneration and they were divided randomly into two groups:
Chitosan preparation
It is prepared in an adhesive in situ-gelling, cell carrier has been developed by mixing four volumes of a viscous chitosan-GP solution with one volume of a hydroxyethyl cellulose (HEC) solution carrying a cell dispersion.18
Surgical procedure
Patients were prepared according to the collected data including radiographic examination, vitality testing, anatomical consideration and the amount of alveolar bone support of the involved teeth. Endodontic treatment performed before operation, and removal of involved hopeless teeth were decided to be extracted during surgery. The operation was done under local anesthesia (Septanest with adrenaline 1:100,000; Septadont, Saint Maur Des Fosses, France) and access to the cystic cavity was gained through incising and reflecting an adequate mucoperiosteal flap. Three-incision pyramidal mucoperiosteal flap was done to allow for adequate access of the operating field with good blood supply. The overlying bone is removed and a thin bladed curette was used to separate the cyst lining from the bone. The bony edges of the defect was saucerized with rongeur and smoothed with bone file before the soft tissues were approximated. And finally the wound was irrigated with sterile saline. In group B, the cystic cavity was kept empty and the wound was closed with three zero black silk sutures. While in group A, the cystic cavity was filled with demineralized lyophilized equine bone matrix (osteoplant activagin bioteck product). The graft material is commercially available in sterilized sealed plastic containers containing 0.5gm of demineralized lyophilized equine bone matrix in particle size of 0.5mm to 1mm. The content of the container is mixed with chitosan and the admixture is packed into the defect. Finally, the wound was closed using three zero black silk suture (Figure 1).
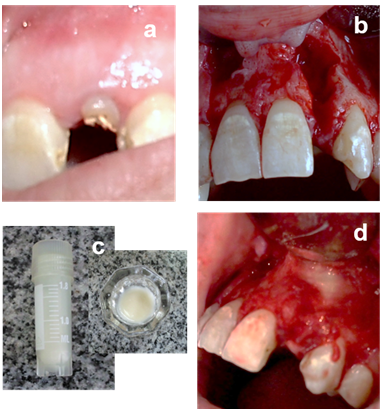
Figure 1
a. Preoperative clinical view.
b. Anterior maxillary cystic defects after reflection of mucoperiosteal flap and complete enucleation.
c. Demineralized lyophilized equine bone matrix (osteoplant activagin) is hydrated with sterile saline.
d. The material is packed into the left defect & right defect as control.
Post operative care and follow up
Following surgery, all patients were given proper postoperative instructions and oral hygiene measures. Augmentin 1gm (2gm/24 hours) was administrated for 5 days postoperatively. Brufen 400mg was prescribed as one tablet whenever needed to control postoperative pain. Sutures were removed after one week in all patients.
Evaluation of repair
Clinical examination: All patients were evaluated postoperatively by repeated clinical examination for the first four weeks and then monthly for six months. Every patient was examined for the clinical condition of the wound, evidence of edema, infection, any discharge or wound breakdown which may suggest complicated healing.
Radiographic examination: The operated site was radiographed using CBCT to determine any changes in radio density. The rate and amount of bone regeneration was assessed by cone beam computed tomography immediately, 3 and 6 months post operatively.
Histologic & histomorphometric evaluation: After healing periods from 5 to 8 months (mean 6 months), reentry surgery was performed and a core bone obtained by trephine drill (10mm in length and 3mm in diameter) from cysts augmentations in all patients simultaneously with implant placement. Biopsies were immediately fixed in 4% formaldehyde for 24 hours at 4°C and decalcified in 10% formic acid for 3days. After dehydration in ascending alcohol series, the biopsies were embedded in paraffin, and 5μm thick sections parallel to the longitudinal axis of the biopsy specimen were prepared using a microtome.
Statistical analysis
Data gained were subjected to descriptive statistical analysis in this study. Numerical data were presented as mean and standard deviation (SD) values. Student’s t-test was used to compare between mean bone density measurements in the two groups. Paired t-test was used to study the changes by time in each group. Gender data (Qualitative data) were presented as frequencies and percentages. Chi-square test was used to compare between the two groups. The significance level was set at P≤0.05. Statistical analysis was performed with IBM® SPSS® Statistics Version 20 for Windows.
All cystic cavities left were 3-wall defects following enucleation of the lesions. Each case had an uneventful postoperative course and was followed, for 6 months in both groups. At the end of the first week postoperatively, uneventful healing was detected in all patients. Patients (both groups) complained of mild pain the day of operation, with tenderness which disappeared by the end of the 1st week. There was no reported cases undergoing infection and the overlying mucosa showed normal appearance in the surgical site. By the end of the 3rd month function are well tolerated and no morbidity was detected and all patients were asymptomatic. By the end of 6th month neither patients complain nor were abnormal findings reported. Teeth included in the cystic cavities showed less degree of mobility.
All implants showed favorable osseointegration, and final restoration was placed without complication or failure (Figure 2). The success of the implant was evaluated by Albrektsson criteria from 1986.20
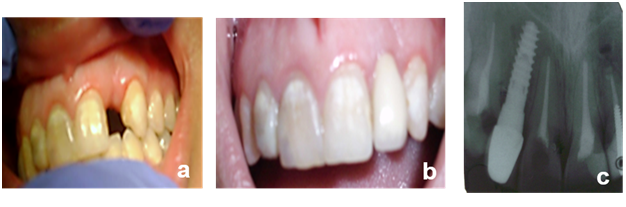
Figure 2
a. Postoperative clinical follow up reveals uneventful healing.
b. Postoperative clinical follow up reveals uneventful healing.
c. Post operative radiograph showing implant in the augmented defect.
Radiographic assessment
All patients were radiographically assessed for bone density at follow up periods (Figure 3). Comparison between bone density measurements in the two groups reveals that at base line and after 3 months; there was no statistically significant difference between mean bone density measurements in the two groups. After 6 months; study group showed statistically significantly higher mean bone density than control group (Table 1 & Figure 4).
Group |
Study |
Control |
P-value |
||
Period |
Mean |
SD |
Mean |
SD |
|
Base line |
238.7 |
60.8 |
243.8 |
26.6 |
0.93 |
3 months |
405 |
74.3 |
322.5 |
82.2 |
0.202 |
6 months |
552.1 |
69.6 |
376.3 |
71.6 |
0.010* |
Table 1 The mean, standard deviation (SD) values and results of Student’s t-test for comparison between bone density measurements in the two groups
*: Significant at P ≤ 0.05
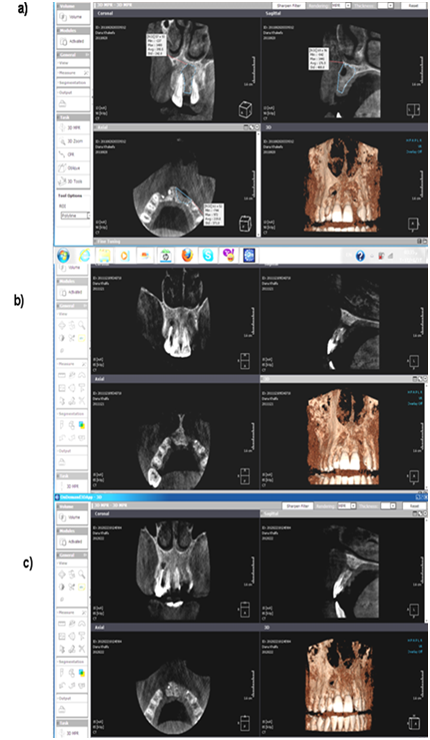
Figure 3 Radiographic assessment by CBCT for case 1 (study & control group):
a. Immediately postoperative,
b. At 3rd month.
c. At 6th month.
As regard changes by time within each group, the Study group, showed statistically significant increase in mean bone density after 3 months and after 6months. While in control group, there was non-statistically significant increase in mean bone density after 3months. While 6month postoperatively, there was statistically significant increase in mean bone density (Table 2, Table 3 & Figure 5).
Period |
Mean |
SD |
P-value |
Base line – 3 months |
166.2 |
61 |
<0.001* |
Base line – 6 months |
313.4 |
72.6 |
<0.001* |
Table 2 The mean differences, standard deviation (SD) values and results of paired t-test for the changes by time in mean bone density measurements of study group
*: Significant at P ≤ 0.05
Period |
Mean |
SD |
P-value |
Base line – 3 months |
78.8 |
58.6 |
0.075 |
Base line – 6 months |
132.5 |
27.2 |
0.002* |
Table 3 The mean differences, standard deviation (SD) values and results of paired t-test for the changes by time in mean bone density measurements of control group
*: Significant at P ≤ 0.05
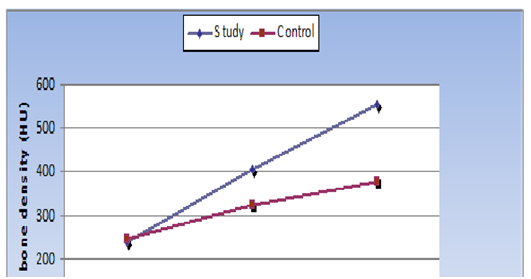
Figure 5 Line chart representing changes by time in mean bone density measurements of the two groups.
Finally, the percentages of increase in bone density in the two groups after 3months showed no statistically significant difference between them. While by the end of 6months study group showed statistically significantly higher mean % increase in bone density than control group (Table 4 & Figure 6).
Group period |
Study |
|
Control |
|
P-value |
|
Mean % |
SD |
Mean % |
SD |
|
Base line – 3 months |
70.3 |
18.3 |
46.8 |
12.5 |
0.294 |
Base line – 6 months |
141.9 |
57.7 |
68.3 |
16.1 |
0.005* |
Table 4 The mean %, standard deviation (SD) values and results of Student’s t-test for comparison between percentages of increase in bone density in the two groups
*: Significant at P ≤ 0.05
Histological & histomorphometrical assessment
In histological analysis, new bone was formed between the grafted bone particle, and the grafted bone was well integrated to the newly formed bone matrix. The maturity of new bone varied from woven to mature lamellar bone. The grafted bone was embedded in newly formed bone, and individual DBM particles were interconnected through trabecula formation. The fibrovascular connective tissue around the new bone and graft materials showed neither inflammation nor foreign body reaction (Figure 7). The total bone volume was 68.9±9.6% and vital bone volume was 25.2±11.9%. The percentages of fibrovascular marrow tissue and nonvital grafted bone were 31.1±9.6% and 43.7 ± 17.2%, respectively (Table 5).
Case No. |
Total bone |
Vital new |
Nonvital Grafted |
Relative Vital New |
Fibrovascular Marrow |
|
Vol. (%) |
Bone Vol. (%) |
Bone vol. (%) |
Bone Vol. (%) |
Tissue Vol. (%) |
1 |
60.4 |
17.3 |
43.1 |
28.6 |
39.6 |
2 |
76.4 |
33.2 |
43.2 |
43.4 |
23.6 |
3 |
76.8 |
25.7 |
51.1 |
33.5 |
23.2 |
4 |
56.8 |
39.7 |
17.1 |
69.9 |
43.2 |
5 |
74.3 |
10.2 |
64.1 |
13.7 |
25.7 |
6 |
76.3 |
34.4 |
42.8 |
43.5 |
23.4 |
Mean ± SD |
68.9±11.9 |
25.2±11.9 |
43.7±17.2 |
37.8±20.9 |
31.1±9.6 |
Table 5 Histomorphometric analysis of bone formation parameters after cyst augmentation with DBM
Masson Trichrome histochemical stain applied to mature normal bone exhibited two main reactions: a blue reaction mainly localized to the osteoid tissue and collagen fibers distribution, and a red reaction for lamellar bone formation (Figure 8).
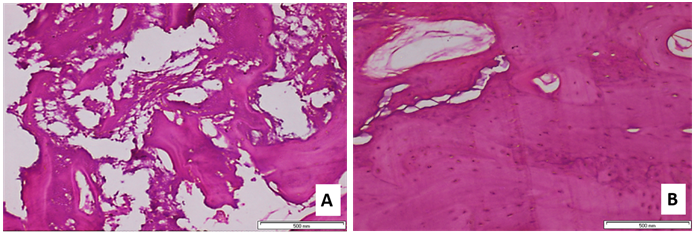
Figure 7
a. Histologic view of core taken at 5 months (Case no. 5). Grafted bone is surrounded by connective tissue without inflammatory reaction, new bone can be observed through some grafted bone surface, and some grafted bone is integrated into new bone (original magnification x200).
b. Histologic view of core taken at 8 months (case no. 4). Complete bone formation can be observed around grafted bone, with a dilated haversian canal and well observed osteoblast cells (original magnification _200).
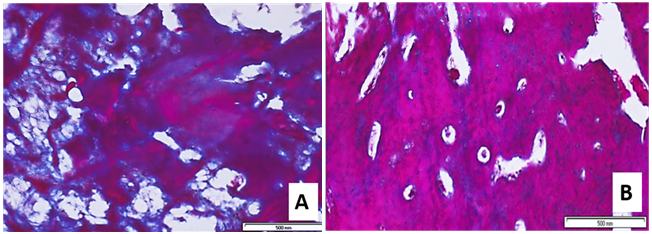
Figure 8 Masson Trichrome histochemical stain:
A) A photomicrograph of group A showing lamellar bone with marked accumulation of connective tissue and osteoid tissue (Masson Trichrome x20).
B) A photomicrograph of group B showing lamellar bone with decreased Masson Trichrome stain distribution and well observed haversian system (Masson Trichrome x20).
The search for the best graft materials for bone regeneration and bone graft substitutes continues. The best substances are those that affect cell activity and have similar biological and biomechanical characteristics to natural bone tissue. These materials are biocompatible, osteoconductive, and osteoinductive, and reinforce bone repair.21,22
Among many bone substitutes available and various techniques tried, with variable rates of success were reported. The selection of the graft material and the choice of the surgical technique are based on the location and extent of the bone loss. The discovery of bone morphogenetic proteins marks a major step forward in the understanding of bone physiology and in the development of advanced methods in skeletal surgery. The efficacy of BMPs as stimulators of bone repair has been demonstrated by many investigators and in the recent study. Bone Morphogenic Proteins have multiple uses in fracture management, including use as an alternative or adjunct to bone grafting in craniomaxillofacial surgeries.23 As an alternative to autograft in long bone nonunion and acute open fractures of the tibial shaft.24 Therefore, the design of the present study, targeted to test the effect of equine demineralized lyophilized bone matrix in grafting human cystic jaw defects in comparison to spontaneous healing of the defects without grafting using cone beam computed tomography.
In fact, DBM is a form of allogenic bone graft substitute material. DBM is composed of insoluble collagen and proteins that are non collagenous in nature and have low concentrations of growth factors.25 The decalcification process that occurs in bone products contributes to the exposition of proteins (collagen and non collagen). This feature gives rise to another activity in bone regeneration. Indeed, the osteoinduction feature of these materials can appear by omitting 90% of calcified materials. DBM has been widely used in different clinical applications in powder form, particles of various sizes, large segments, gels, putties, and other composites. The primary osteoinductive component of DBM is a series of low-molecular-weight glycoproteins, which includes bone morphogenetic proteins (BMPs).26-28 In addition to the inductive effect of the proteins in DBM, the collagen structure plays an osteoconductive role and supports new bone formation.27,28 DBM has a number of additional advantages that make it an attractive bone graft alternative. It is cost-effective and readily available from the tissue banks. The demineralization process destroys the antigenic materials in bone, making DBM less immunogenic than mineralized allograft.28
Therapy with bone morphogenetic proteins (BMPs) has shown potential as a clinically useful alternative to autogenous bone grafting. They were able to repair bony defects in various aspects. Clinical trials have shown the efficacy and safety of recombinant human bone morphogenetic protein-2, for augmentation and preservation of the alveolar bone also, in long bone fractures. Recombinant human bone morphogenetic protein-2 has shown a progress in orthopedic and in maxillofacial fields.29,30 Indeed, our results have got a good impression with filling defects with equine bone morphogenic proteins in critical sized cystic defects (less than 3cm in diameter).
Further success has been reported in our work and was in accordance with,31 who had performed an onlay apposition of equine bone covered by a titanium-reinforced membrane, for augmentation of horizontal mandibular ridge defects, with no post-operative complications occurred. At test sites horizontal bone width increased compared to control sites.31 This study revealed a statistically significant increase in mean bone density of the grafted sites by (313.4) after 3- 6 months from filling the cystic cavities compared to non-grafted group by (132.5) which wassignificant at P ≤ 0.05.
Our successful augmentation of jaw defects with an alternative bone filling materials such as equine BMP was confirmed by previous work made by,32 who grafted cystic cavities with mixtures of autogenous and xenogenic after multiple cystic enucleation in the right upper posterior maxilla and the left lower posterior mandible.32 Also, xenografts of Kiel bone in combination with autologous aspirated bone marrow were used to graft 20 cystic defects in the jaws. Sixteen cases (80%) were successful. Cases were evaluated clinically and radiographically for 4 years after surgery.33
The augmentation of bone defects in oral cavity using DBM exclusively was reported by Kuvat et al.34 and Sohn et al.35 Both, authors presented an increased bone volume in combination with Le Fort I osteotomy and maxillary sinus augmentation. However, no bone development could be achieved in periodontal surgery using DBM alone. Regarding, our study, by the end of 6 months, the study group showed statistically significantly higher mean percentage increase in bone density than the control group.
Regarding the spontaneous healing of the cystic defects without the use of any grafts,36 studied bone healing in cavities after cyst removal for two groups of 50 patients. The control group showed spontaneous bone deposition while, in the test group, where bovine collagen derivative was used. They demonstrated that bovine material was resorbed with delayed ossification in the test group. Also,37 have used autogenous, allogenic, xenogenic and alloplastic bone grafts and compared the results to current investigations about conservative cyst enucleation without using any filling materials. They concluded no superiority of additional bone grafts to the cystic defects.36,37 These findings were in accordance with ours, since the control group has shown significant increase, in mean bone density at the end of the study period, without the use of any grafts, compared to increased bone density in the study group. Also,38 investigated the beneficial effects of DBM and reported no significant differences between the treatment group and the control group. But the results of our work showed that there was statistically significantly mean percentage increase in bone density of study group than control group at the end of 6 months (141.9% and 68.3% respectively).38,39 Also histological analysis revealed that the total bone volume was 68.9±9.6%, and vital bone volume was 25.2±11.9%. The percentages of fibrovascular marrow tissue and nonvital grafted bone were 31.1 ±9.6% and 43.7± 17.2%, respectively.
In accordance with the work done by Pradel et al.,40 they treated 22 mandibular cysts with bone grafts. Eleven cysts were filled in with autogenous osteoblasts cultured on demineralized bone matrix (Osteovit) and another 11 cystic defects were filled with spongiose iliac bone as controls. In both groups bone regeneration took place in a similar fashion. After 3 and 6 months there were few differences in bone density between the groups. However, in radiographic controls after 12 months ossification was considerably stronger in cysts grafted with tissue-engineered bone. They advocated the clinical application of tissue-engineered bone as an alternative viable filling material for cysts.40
Demineralized bone matrix (DBM) powder is widely used for bone regeneration due to its osteoinductivity and osteo conductivity. However, difficulties with handling, its tendency to migrate from graft sites, and lack of stability after surgery can sometimes limit the clinical utility of this material. In this work, the using of a thermogelling chitosan carrier to deliver DBM powder was utilized. The DBM-thermogelling putty improved handling and formed a gel-like composite in situ at body temperature within a clinically relevant time period. The properties of the formed composite, including morphology, porosity, mechanical properties, equilibrium swelling as well as degradability, are significantly influenced by the ratio of DBM to thermogelling chitosan.41
Collagen is a member of major importance in the extracellular matrix bone tissue. It also changes according to the physiologic state of bone, as any alteration in its distribution can be indicative for the different changes in bone tissue during the study.42 In the present study, the increased amount of osteoid tissue in addition to the increased amount of calcified tissue in the study group reflects accelerated bone remodeling and effective therapy. Moreover, the presence of thin osteoid layer covering most of the peripheries of bone trabeculae in the study group is a sign for continuous remodeling this was in accordance with Loveridge.43
In this study, cystic cavity augmentation was performed by using DBM. In all of the patients, the histologic results showed well formed new bone without inflammation during follow-up period. Also, DBM-chitosan admixture is easy to handle without scattering, and this property can facilitate the retention and slow release of BMPs at the surgical site, which can enhance osteoinduction. We concluded that DBM is clinically useful in bone defects augmentation for favorable new bone formation and easy handling.
We would also like to show our gratitude to the (Dr. Layla Omara, Professor of Oral and Maxillofacial Surgery, Cairo University) for sharing her pearls of wisdom with us during the course of this research, and her comments on an earlier version of the manuscript, although any errors are our own and should not tarnish the reputations of these esteemed persons.
The author declares that there was no conflict of interest.

©2015 Aly, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.