Journal of
eISSN: 2574-9943


Case Report Volume 3 Issue 2
1Faculty of Medicine, South Santa Catarina University (UNISUL), Brazil
2Professor Ruben David Azulay Dermatology Institute, Santa Casa da Miseric
3Private Dermatology Clinic, Brazil
4Pathology Post-Graduation Program
Correspondence: Gustavo Moreira Amorim, Faculty of Medicine, South Santa Catarina University (UNISUL), Brazil, Tel +5548 9998 2709 7
Received: February 19, 2019 | Published: March 6, 2019
Citation: Pazian GM, Wessler PG, Amorim G, et al. Photosensitivity and hyperpigmentation secondary to amiodarone: single case report. J Dermat Cosmetol. 2019;3(2):32-34. DOI: 10.15406/jdc.2019.03.00111
We report and emblematic case of a 65years-old male patient with cutaneous photosensitivity and progressive hyperpigmentation of photo exposed skin after continuous use of amiodarone to treat an atrial fibrillation developed along with chronic obstructive pulmonary disease. Relevant literature was consulted and is discussed together with the findings of the case.
Keywords: amiodarone, dermatology, photosensitivity, phototoxic reactions, hyperpigmentation
Amiodarone is an iodine-rich antiarrhythmic agent that is widely used in the control of ventricular and supraventricular arrhythmia, and is superior to other antiarrhythmic drugs for maintenance of sinus rhythm.1,2 It can be used both in the acute treatment of sustained ventricular tachyarrhythmia’s regardless of hemodynamic stability, as in the chronic treatment of patients with left ventricular dysfunction and heart failure; it also serves as prophylaxis in the perioperative period of cardiac surgery and as adjuvant of the implantable cardioversor-defibrillator to reduce the number of shocks.3,4
Amiodarone can cause a large range of side effects due to toxicity to various organs and systems when used for long periods, particularly in high doses. The possibility of thyroid alterations, besides ocular, pulmonary, hepatic and cutaneous toxicities are highlighted.3,5 The most common side effects are bradycardia, hypotension, visual acuity disorders, nausea, intestinal constipation, phlebitis and thyroid changes.6,7
The incidence of cutaneous side effects is high and reaches 75% among patients in chronic and prolonged use of amiodarone. The most common are photoallergic and phototoxic reactions in addition to hyperpigmentation. Less commonly, urticaria, pruritus, erythema nodosum, purpura and erythema multiforme may occur. Rare effects are described as: Stevens Johnson syndrome and toxic epidermal necrolysis, IgA bullous dermatosis and pseudoporphyria. Some reports mention the potential carcinogenic effect of amiodarone, such as the appearance of basal cell carcinoma at sites of previous photosensitivity/phototoxicity.8‒11 We report a case of a male patient with atrial fibrillation who presented a classic and exuberant reaction of photosensitivity and hyperpigmentation, discussing these findings with what is reported in the literature.
A 65-years old male patient, former smoker with a chronic obstructive pulmonary disease (COPD), during a hospitalization for acute lower respiratory tract infection evolved with tachycardia, palpitation, chest pain and respiratory failure. Electrocardiogram showed atrial fibrillation with rapid ventricular response. There was no hemodynamic instability. Patient was treated with chemical cardioversion with intravenous amiodarone, in addition to broad-spectrum antibiotics and therapeutic measures for COPD.
After complete improvement, patient was discharged receiving as a continuous prescription, daily dose of amiodarone. Approximately 2 to 3months after hospitalization, patient complained of increased skin sensitivity when exposed to the. He reported erythema and burning sensation of the skin even after short periods of sun exposure. The lesions cleared quickly with the use of moisturizer. He had an initial evaluation, having been medicated only with sunscreen. As the time went by, photosensitivity was maintained, and even intensified, and the lesions did not clear any more, but remained with residual hyperchromia.
One year after hospitalization, he was referred to out clinic. Dermatological exam showed an intense grayish brown hyperpigmentation of the skin over photo exposed areas, denoting clear photosensitivity. Covered areas were spared (Figures 1). Patient has been using amiodarone ever since. With the hypothesis of an amiodarone cutaneous side effect, an evaluation with the cardiologist was requested. It was recommended the suspension of the amiodarone and initiation of propafenone. In a recent consult with 3months follow up after suspension, patient notice a reduction of photosensitivity symptoms and slight lightening of hyperchromia.
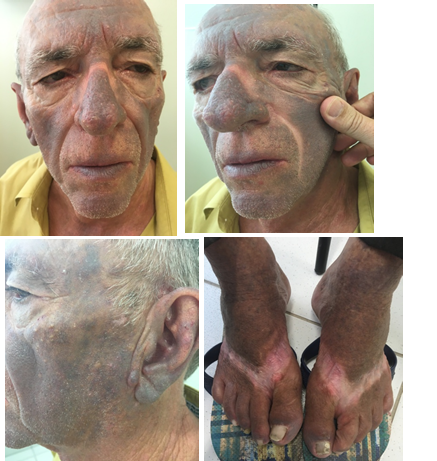
Figure 1 Dermatological exam showed an intense grayish brown hyperpigmentation of the skin over photo exposed areas, denoting clear photosensitivity. Covered areas were spared.
The photosensitivity caused by amiodarone ranges from an increased capacity to tan to a burning sensation or itching at sites exposed to radiation, often accompanied by erythema.10 Clinical features of the photosensitivity response represent a phototoxic reaction to amiodarone and its major metabolite, mono-N-desethylamiodarone.11 Approximately 50% of all amiodarone-treated patients develop symptoms of acute photosensitivity, usually after 4months of continuous treatment and a minimal dose of 40g.11‒14 Photosensitivity gradually declines and returns to normal 4 to 12months after discontinuation of amiodarone therapy12. However, it can sometimes last several years after drug withdrawal.10
Amiodarone-related hyperpigmentation occurs due to skin drug deposition. Study conducted by Ammoury et al.11 published in 2008, identified the presence of high iodine concentrations, which was observed in the electronic probe analysis, suggesting that cutaneous deposits are composed of amiodarone itself or a metabolite. Therefore amiodarone deposits in the skin, with or without lipofuscin, are capable of inducing gray-blue hyperpigmentation. Cutaneous hyperpigmentation develops mainly in patients with Fitzpatrick's skin type, with an incidence of approximately 10% of treated patients.11 It occurs after an average of 20months of continuous use and a minimal cumulative dose of 160g.12 The main treatment of both photosensitivity and hyperpigmentation is amiodarone suspension, in cases where another antiarrhythmic agent can replace its use. When it’s not possible, dosage should be reduced to the minimum necessary to control cardiologic comorbidity, reducing deposition and contributing to faster tissue clearance.15 Additionally it should be recommended daily use of broad spectrum sunscreen, preferably with zinc oxide-containing preparations and avoid sun exposure.14 Other therapeutic options include chemical peels, narrow band UVB, Q-switched ruby laser.16,17
The authors present this emblematic case with good photographic documentation in order to make a point of the possible adverse effects inherent to amiodarone, a widely used antiarrhythmic drug. Adverse cutaneous manifestations related to its prolonged use are common and should be known by general practitioners. Although rarely serious, they determine an important impairment of patients' quality of life. Once identified and correctly diagnosed, suspension of the drug allows the improvement of the picture in most cases. However, the discontinuation of amiodarone should be discussed in a multidisciplinary team, considering its risks and benefits.
None.
Authors declare that there are no conflicts of interest.

©2019 Pazian, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.