Journal of
eISSN: 2574-9943


Case Report Volume 3 Issue 2
1Dermatology Division, Department of Medical Specialties, Saudi Arabia
2Department of Radioimaging, Saudi Arabia
3Department of Pathology, Saudi Arabia
Correspondence: Taseer Ahmed Bhatt, Dermatology Division, Medical specialties Department, King Fahad Medical City, Dabab Street, Riyadh; Saudi Arabia-11525, PO Box: 59046, Tel +966-11-2288 9999
Received: September 13, 2017 | Published: April 1, 2019
Citation: Bhatt TA, Daghriri A, Shakweer WA, et al. Neurocutaneous melanosis with pulmonary metastasis: looking beyond the skin. J Dermat Cosmetol. 2019;3(2):42-44. DOI: 10.15406/jdc.2019.03.00113
Neurocutaneous melanosis are neuroectodermal disorders characterized by giant congenital melanocytic nevus or multiple (≥3) nevus’s associated with melanocytic leptomeningeal tumour. Central nervous system tumour can be benign or malignant and usually presents at an early age. The neurological manifestation includes raised intracranial pressure, seizures and spinal cord compression. The dissemination of malignant nevomelanocytes to the peritoneal cavity has been observed with ventriculoperitoneal shunt placement in neurocutaneous melanosis. We report an infant of neurocutaneous melanosis with primary central nervous system melanoma and its metastasis to the lung in view of rarity.
Keywords: neurocutaneous melanosis, quadreparesis, metastasis
Neurocutaneous melanosis is a rare congenital disorder characterized by giant congenital melanocytic nevi or multiple (≥3) nevi and abnormal deposits of melanin in the brain and leptomeninges. The letomeningeal tumours are at high risk of malignant transformation and are associated with an aggressive course. The dissemination of the malignant nevomelanocytic cells to the peritoneum been known to occur through ventriculoperitoneal shunt but metastasis to the lungs has never been reported in the literature.
What is known?
Neurocutaneous melanosis with neurological symptoms have poor prognosis independent of benign or malignant nature of the leptomeningeal tumour. The dissemination of melanoma to peritoneum through the ventriculoperitoneal shunts have been reported in literature.
What is new?
The presence of pulmonary metastasis has not been reported in the literature.
4 months old female infant was brought by her mother to the pediatric emergency room of King Fahad Medical City, Riyadh. The infant had large hyperpigmented patches with superimposed nodular lesions involving the head and entire trunk. There was no parental consanguinity. She was born in a hospital at full-term by normal spontaneous vaginal delivery with Apgar score of 9 and weighed 2.4kg at birth. At the age of one month, the infant began to have seizures. The patient was seen in a tertiary care hospital in Aseer and on imaging studies was diagnosed to have congenital hydrocephalus. She was treated for hydrocephalus with ventriculoperitoneal shunt placement and was started on anticonvulsants. The infant continued to have poor control of her seizures and was recently noted to have decreased limb movements.
On general physical examination the baby vital signs were stable with oxygen saturation of 100% on room air. The baby weighed 3kg and had aphonic cry. The systemic examination was normal except for neurological examination which showed evidence of quadriparesis. The cutaneous examination revealed large brown to black pigmented confluent patches, plaques and multiple nodular masses involving the scalp, neck and entire back associated with hypertrichosis. The surface of the nodular masses had rogues appearance. The lesions were soft on palpation and some of the nodular lesions showed erythema and erosions with whitish discharge (Figure 1). There was no involvement of oral mucosa and ophthalmologic examination was also normal. Patient was admitted for further evaluation in the pediatric hospital of King Fahad Medical City.

Figure 1 Multiple large brownish black pigmented patches, plaques and nodular masses on the scalp, neck and back with hypertrichosis.
Baseline laboratory work up was done and was normal. Skin biopsy was done which showed intradermal proliferation of melanocytes and melanin incontinence. There was no evidence of atypia or mitosis (Figure 2). Her chest X-ray and ultrasound abdomen was a normal. Magnetic resonance imaging of brain and spine was done and revealed shunted ventricular system, dilated lateral and 3rd ventricle. There were large deposits in the right cerebellopontine angle and throughout the spinal canal (Figure 3& Figure 4). The cerebrospinal fluid examination was done and revealed few melanin laden macrophages (Figure 5). CT scan of trunk, abdomen and pelvis showed evidence of metastasis at the apical lobe of right lung with cavitation (Figure 6). Neurosurgical consultation was done but in view of the extent of brain and spinal involvement, it was decided against surgical intervention. Pediatric oncology team was involved and patient was started on interferon. During the hospital stay the baby developed flaccid quadriplegia and bulbar palsy. Her condition deteriorated further due to aspiration pneumonia followed by sepsis. She was shifted to pediatric intensive care unit where she had cardiorespiratory arrest and did not respond to resuscitation measures.
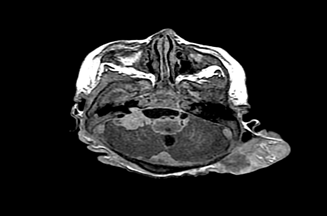
Figure 3 Axial T1 post contrast with intracranial metastatic lesion at the right posterior fossa and leptomeningeal deposits, left occipital scalp mass is noted.
Neurocutaneous melanosis (NCM) is a rare congenital sporadic disorder characterized by abnormal migration and proliferation of the precursor melanocytes from the neural crest to the skin and leptomeninges. In some cases of NCM, the abnormal melanin deposits are seen to involve the brain parenchyma as well as the spinal cord.1 The disorders presents in infancy as congenital giant melanocytic nevus or multiple nevus's(≥3) involving the lumbosacral area. The giant or large congenital melanocytic nevus in neonates and infants is defined as size ≥9cms on head or ≥6cms on the body.2 The imaging studies have revealed asymptomatic presence of neural involvement in approximately 25% of such cases.3 The presence of neurological symptoms in infancy indicates a poor prognosis.2
The most common neurological complication of NCM is hydrocephalus as was seen in our case. The etiology of hydrocephalus is thought to be mal-absorption of cerebrospinal fluid secondary to melanocytic infiltration of arachnoid villi and obstruction of cisterna magna by the deposits of melanin.4 Other signs of neurological involvement in NCM include irritability, lethargy, emesis, seizures, and cranial nerve palsies.5 NCM can affect the amygdala, cerebrum, cerebellum, pons, medulla and the spinal cord. The spinal involvement is seen in 20% of cases resulting in myelopathy, radiculopathy, bladder and bowel dysfunction.6 Dandy-walker malformation has been described with NCM and is associated with poor prognosis.7 The most serious complication is degeneration of abnormal neural melanocyte proliferation to melanoma. The progression of neural melanosis to melanoma has been reported in approximately 50% of the cases.2 However, regardless of the presence or absence of malignancy, the majority of patients die within 3years of initial neurological presentation.8
Our patient presented with extensive congenital giant melanocytic nevi involving the head and most of the trunk with proliferative nodular lesion. The seizures occurred very early in life and were followed by onset of quadriparesis at 4months of age. There are very few case reports in the literature were NCM presented as seizures within first month of life and followed such aggressive course.9 This rapid deterioration in our patient indicated a malignant transformation. The presence of a necrotizing nodule in the right lung on CT scan suggestive of metastasis further supported the presence of melanoma. There are case reports of dissemination of the CNS melanoma to the peritoneal cavity through the ventriculoperitoneal shunts.10,11 Neurocutaneous melanosis with hepatic metastasis has also been reported with primary in the cerebellum.12 The presence of pulmonary metastasis in NCM to the best of our knowledge has never been reported in the literature.
The cutaneous lesions in comparison to central nervous system nevomelanocytic proliferation do not progress to melanoma at an early age as has been reported in the literature.13 Our patient also did not show any evidence of malignancy on skin biopsy. Dermatologists are the first specialty consulted in view of the presence of the congenital melanocytic nevus. They should be aware of the possibility of NCM in all patients presenting with single congenital melanocytic nevi of large size or smaller multiple (≥3) nevi involving the head and trunk. The MRI study of brain and early referral to pediatric neurosurgeon is advisable. However there is no surgical or medical treatment available at present in NCM except for palliative care. Parents should be counseled about the diagnosis and prognosis of neurocutaneous melanosis.
None.
The authors declare no conflicts of interest.

©2019 Bhatt, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.