Journal of
eISSN: 2574-9943


Case Report Volume 2 Issue 1
1Department of Dermatology, FAMERP, Brazil
2Department of Pathology, Instituto de Anatomia Patológica, Brazil
Correspondence: Lívia Arroyo Trídico, Department of Dermatology, FAMERP, Rua Silva Jardim 3114, Centro, São José do Rio Preto, São Paulo, Brazil, Tel 17)3232-6611
Received: August 22, 2017 | Published: January 9, 2018
Citation: Mendes AM, Antonio JR, Pozetti EMO, et al. Lichen myxedematosus: atypical form and therapeutic success with intravenous immunoglobulin. J Dermat Cosmetol. 2018;2(1):1-3. DOI: 10.15406/jdc.2018.02.00025
Background: Lichen myxedematosus atypical is an unusual type of lichen myxedematosus. Its treatment is difficult and undefined due to the rarity and the shortage of cases described in the literature.
Objective: Report a case of atypical lichen myxedematosus treated effectively using immunoglobulin.
Methods: A 53-year-old female patient diagnosed with atypical lichen myxedematosus without response to previous treatment with systemic corticosteroids, thalidomide, phototherapy and methotrexate was treated with intravenous immunoglobulin at a dose of 0.4g/kg/day in 5day cycles with an interval of 30 days between them.
Results: The patient presented a clear improvement in the first month of treatment, and after six months the most part of the cutaneous lesions was solved with maintenance of results during one year of treatment and clinical follow-up.
Conclusion: In this study it was possible to verify therapeutic response to immunoglobulins in a patient with rare manifestation of atypical lichen myxedematosus.
Keywords: scleromyxedema, therapeutics, immunoglobulins, IV, gammopathy
Lichen myxedematosus is a cutaneous mucinosis of idiopathic origin. According to Rongioletti's classification, 2006, lichen myxedematosus is classified into three forms. The scleromyxedema or lichen myxedematosus sclerodermiform and generalized is a form of lichen myxedematosus characterized by numerous papules and areas of cutaneous hardening due to the deposition of mucin in association with the increase of dermal collagen, in addition to monoclonal gammopathy and systemic manifestations that can be lethal. Another form of lichen myxedematosus is the localized one, distinguished by the presence of papules and cutaneous hardening in certain areas of the skin (sub classified into four subtypes: discrete papular form, acral persistent papular mucinosis, cutaneous mucinosis of infancy and pure nodular form). The third form is classified as atypical or intermediate and is characterized by clinical features of scleromyxedema but without monoclonal gammopathy, or also localized forms with monoclonal gammopathy and/or systemic symptoms.1-3
The treatment of scleromyxedema is difficult and undefined, thus several therapeutic options are used resulting into disappointing outcomes: high doses of corticosteroids, immunosuppressive and chemotherapeutic drugs, phototherapy, plasmapheresis, retinoids, thalidomide, immunoglobulins, among others.4 In general, the localized forms of lichen myxedematosus use topical treatments with corticoids.1 The treatment for atypical forms is even more indefinite and difficult due to their rarity and the shortage of cases described in the literature. In this article, we shall report a case of atypical lichen myxedematosus treated effectively using immunoglobulin.2,4
A 53-year-old female patient reported an acute and progressive case of cutaneous edema and hardening of the face, auricular pavilions and hands that started a week before the appointment, associated with an intense pruritus. Such case evolved with the appearance of papules in the nape of the neck, scalp, retroauricular and axillary regions and anterior chest, in addition to a thickening of the tongue. The dermatological examination revealed a bilateral eyelid edema, associated with the difficulty of ocular opening and nodules located below the eyelids, skin with bright aspect, erythematous-brownish colour and edema in the face, neck and hands regions, and also lichenoid papules in the nape of the neck, neck, scalp, upper chest and back of the hands (Figures 1 & 2).
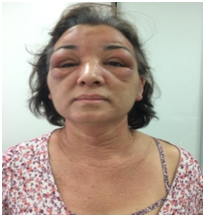
Figure 1 Eyelid edema, infra-eyelid nodules, facial edema and lichenoid papules in the neck and chest.
We performed a cutaneous biopsy in the lesions that showed deposits of mucin in the superficial dermis (alcian blue color) and areas of collagen sclerosis in the deep dermis (Figure 3), thus contributing to the diagnosis of cutaneous mucinosis. Laboratory tests were carried out in order to investigate paraproteinemia due to the clinical background of scleromyxedema. However, the immunoelectrophoresis of proteins did not demonstrate any monoclonal protein. Moreover, laboratory tests such as hemogram, thyroid, hepatic and renal functions, glucose, CPK, DHL, aldolase, calcium, C3 and C4 were normal. Sorologies for viral hepatitis and HIV were negative and the ANA was non-reactive. Protein electrophoresis evidenced the decrease of albumin and increase of alpha 1 and alpha2 globulins. A hematologic investigation was performed with a bone marrow biopsy and no significant alterations were found; imaging tests (ultrasound of the abdomen, face magnetic resonance, x-ray of the hands, elbows, knees and thorax) were also deemed as normal. Therefore, the patient was diagnosed with atypical lichen myxedematosus.
Technique
The treatment was initiated with systemic corticosteroids, prednisone at a dose of 40mg per day, which was maintained for one month, resulting into a very slight improvement of the clinical picture and of the intense pruritus refractory to antihistamines. After that, we reduced the corticosteroid dose to the suspension of it, and introduced the thalidomide at a dose of 100mg per day. The patient presented a discrete improvement after two months of such treatment. Therefore, thalidomide was maintained and associated with phototherapy with UVA and methotrexate at a dose of 25mg per week with no further improvement after two months. Finally, we chose to introduce the immunoglobulin after a literature review on lichen myxedematosus therapy.4-10
The intravenous immunoglobulin at a dose of 0.4g/kg/day was initiated and maintained for 5 consecutive days every 30 days. The patient presented an clear improvement in the first month of treatment, and after six months the most part of the cutaneous lesions was solved, with only discrete papules remaining in the chest region. At present, such patient has performed 14 treatment cycles with monthly intervals and is extremely satisfied with the results. The patient keeps a monthly clinical follow-up, followed by a laboratory investigation for generalized disease, which remains negative (Figure 4).
The treatment of scleromyxedema with doses of intravenous immunoglobulin (IV) has been recently described with promising results, presenting a safe and favorable profile in relation to other therapeutic options.4 Recent studies evaluating the efficacy of scleromyxedema treatment with immunoglobulin IV demonstrate an initial response that is dramatic to therapy, although it is necessary to maintain a long-term therapy in order to achieve the expected results.2 In the study by Blum et al.,5 ten patients diagnosed with scleromyxedema were treated with immunoglobulin IV and showed a remarkable clinical, laboratory and radiological response.5 Moreover, this study revealed that the monoclonality of the patients was not reduced by treatment with immunoglobulin and that the therapeutic cycles were able to maintain the resolution of the cutaneous and extracutaneous disease, however the conservation of the infusions was necessary in order to maintain the clinical response to the treatment.2,5,11
It is believed that immunoglobulin may act by blocking Fc receptors (immunoglobulin receptors), rendering the phagocytic cells nonfunctional.5 Other studies claim that intravenous immunoglobulin "neutralizes pathogenic antibodies through idiopathic antibodies and anti- idiopathic antibodies," affecting dendritic cells and the complement pathway. Some studies also believe that intravenous immunoglobulin alters the metalloproteinases, resulting in a change in the collagen matrix. In case of block of Fc receptors by immunoglobulins, this could explain why paraproteinemia it is solved with the treatment and the need for continuous infusions to maintain the resolution of lesions.2,11-13
In the case presented above, we observed the dramatic response to the treatment within the first month after intravenous therapy, and we maintained the monthly immunoglobulin cycles to maintain the therapeutic response. Differently from the cases reported in the literature to date, our patient did not present a diagnosis of scleromyxedema, since mucin deposition was restricted to the skin and the paraproteinemia was not evidenced. However, in view of the clinical picture of atypical lichen myxedematosus with exuberant cutaneous lesions and little response to the other therapeutic options, we introduced the intravenous immunoglobulin.
It is still needed much more researches regarding lichen myxedematosus, whether sclerodermiform and generalized (scleromyxedema) or not, and also in relation to treatment with immunoglobulins, once it is crucial to define the disease etiology and the mechanism of action of immunoglobulins. Based on previous studies, we know that in cases of scleromyxedema, the intravenous immunoglobulin is well tolerated and effective.
In this study it was possible to verify therapeutic response to immunoglobulins in a patient with rare manifestation of atypical lichen myxedematosus and the maintenance of the results during one year of treatment and the clinical follow-up.
The patient consented to relate her case and gave permission to photographies.
Ana Maria Mendes: Intellectual participation in propaedeutic and therapeutic conduct of the case, patient follow-up and literature review.
João Roberto Antonio: Intellectual participation in propaedeutic and therapeutic conduct of the case and patient follow-up.
Eurides Maria de Oliveira Pozetti: Intellectual participation in propaedeutic and therapeutic conduct of the case.
Lívia Arroyo Trídico: Literature review, manuscript elaboration and manuscript writing.
Thalita Marçal Machado: Literature review and patient follow-up.
Jorge Alberto Thomé: Anatomopathological study and biopsy diagnosis.
The authors declared that there are no conflicts of interest.

©2018 Mendes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.