Journal of
eISSN: 2574-9943


Research Article Volume 1 Issue 2
IPclin Instituto de Pesquisa Clinica Integrada Ltda, Brazil
Correspondence: Leila David Bloch, IPclin Instituto de Pesquisa Clinica Integrada Ltda, 314 Rua Leonardo Cavalcante, 13201-013, Jundiai - SP, Brazil, Tel 55 11 4087-0092
Received: February 21, 2017 | Published: April 28, 2017
Citation: Bloch LD, Escudeiro CC, Sarruf FD. Efficacy assessment of a nutraceutical with a marine protein complex in the reduction of female telogen effluvium. J Dermat Cosmetol. 2017;1(2):33-37. DOI: 10.15406/jdc.2017.01.00008
Introduction: Telogen Effluvium may be related to the deficiency of certain nutrients such as iron, zinc, vitamins, essential fatty acids and proteins. Therefore, nutraceutical products could be used to treat potentially reversible Telogen Effluvium. In this work, the efficacy in increasing the number of hair fibers and reducing hair loss of a nutraceutical product containing proteins, marine extracts and a silica compound (ViviScal®) was assessed. The study also aimed at the subjective evaluation of the product regarding the increase of hair and nails growth, improvement of skin appearance and quality of life, in general.
Materials and methods: The controlled, monocentric study was conducted during 6 months in volunteers with Telogen Effluvium. The efficacy assessment was performed by phototrichogram and by participants’ opinion based on standardized questionnaires.
Results: There was a reduction in the number of telogen hair fibers and an increase in the number of total hair fibers in 83% of the participants after 6 months of treatment. The majority noticed an improvement in hair and nails growth and in skin appearance after 6 months of treatment.
Conclusion: The product was effective in increasing the total number of hair fibers and reducing Telogen Effluvium. This nutraceutical product was also positively evaluated by the participants regarding hair and nail growth, and skin appearance.
Keywords: nutrition, hair loss, phototrichogram, clinical trial
Hair loss is a common complaint in dermatology clinics. The hair cycle is a complex biological process by which human hair follicles undergo three main stages: growth (anagen phase), apoptosis -driven regression (catagen phase) and the quiescent state (telogen phase).1 Telogen Effluvium can affect any age group and has several triggering factors. Among the main causes, can be mentioned: post-partum, thyroid diseases, stress, anemia and certain drugs, such as antineoplastic agents, among others.2-5
Telogen Effluvium can also be related to nutritional deficiencies such as iron, zinc, vitamins, essential fatty acids and proteins.2-4,6 Thus, nutraceuticals can be used in the treatment of such potentially reversible Telogen Effluvium. A complex consisting of proteins, marine extracts and a silica compound (ViviScal®) has been studied for the treatment of hair disorders and showed efficacy in the treatment of androgenetic alopecia.7,8 Increasing in hair growth was also detected by digital photographs and self-assessment questionnaires of volunteers who took oral supplementation with Viviscal®.9
The aim of this study was to investigate the efficacy of a nutraceutical containing the marine protein complex (Viviscal®) in the treatment of Telogen Effluvium in female patients after a 6 months treatment, employing the phototrichogram technique, an objective method to evaluate hair scalp,10 and by the application of standardized questionnaires to assess the opinion of the participants.
Study design
The study was controlled, monocentric, with duration of 6 months and was performed at Instituto de Pesquisa Clínica Integrada Ltda (IPclin Ltda), located in Sao Paulo - Brazil. The procedures were in accordance with ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration.11,12
Research participants’ selection
Fifty-two female volunteers aged between 25 and 50 years, with phototypes II to IV13 were selected to participate in the study after signing the Informed Consent Form. The inclusion criteria were: Telogen Effluvium detected by clinical dermatological evaluation (positive traction test, moderate or strong); hair loss complaint; hair washing at least three times a week and healthy scalp skin. The adopted exclusion criteria were: Androgenetic Alopecia; use of hairpieces; history of allergy or irritation to the product’s formula components; pregnancy; lactating; immunodeficiency; volunteers with kidney, heart or liver transplant; participants that performed hair straightening or relaxation procedures until 3 months before the study beginning; use of systemic or topical medications such as corticoids, anti-histamines, immunosuppressant, retinoids or medications that may interfere with the hair loss cycle. The participants were prohibited to alter diet, exercise and hygiene habits, as well as perform any procedure that may interfere with the hair loss cycle (e.g.: hair dye, straightening, etc.) and to use other products from the assessed category during the study.
Investigational product and posology
The nutraceutical investigational product Viviscal® Maximum Strength Oral Supplement (Lifes2good, Inc., Chicago, Illinois) was given to the volunteers, in a previously disguised packaging, to be used at home during 6 months. The participants were oriented to use two tablets a day during the main meals. Table 1 contains the product Viviscal® composition.
Components |
Amount |
Accerola [Malphighia glabra] Fruit Blend Powder (25% Vitamin C) |
33.33mg |
Ascorbic Acid |
12.0mg |
Marine Protein Complex |
450.0mg |
Horsetail Extract Powder |
45.0mg |
Millet Dry Extract |
7.50mg |
D-biotin |
0.014mg |
Zinc Oxide |
3.90mg |
Ferrous Fumarate |
10.2mg |
Nicotinamide |
6.20mg |
Table 1 Qualitative and quantitative composition of the investigational product Viviscal® Maximum Strength Oral Supplement.
Assessments
The determination of the efficacy of reducing hair loss was performed by questionnaire application to the participants and by phototrichogram. The assessments were performed in the inclusion day, and after three and six months of treatment. Medical assessments of clinical signs and questioning of discomfort sensations referred by the participants were performed every month during the study.
Questioning of the participants
The volunteers were questioned about the impact of hair appearance on their self-esteem and life quality at the beginning of the study (D0) and after 6 months of treatment (D180). Besides, they responded to a self-assessment questionnaire after 3 (D90) and 6 months of product usage (D180). The questions and answers of volunteers are presented in Table 3.
Phototrichogram
An area of approximately 2 x 1cm was selected on the right or left frontoparietal region of each volunteer, by the dermatologist. Then, the hair of the selected area was shaved until the shortest length possible. This shaving procedure was performed monthly to allow the analysis always to be performed on the same region along the study. After shaving, the region was photographed with a camera (Canon, model T3i) coupled with an optical accessory lens provided with own lighting. Two days after, the site was again photographed. This procedure was repeated after 3 and 6 months of treatment.
The images obtained at each experimental time were treated and analyzed by the program Image Pro Plus®, version 6.0, for total and telogen hair counting. The counting performed by the program was compared to the one performed manually by the dermatologist for results’ validation. The product efficacy was determined based on the comparison of the total and telogen hair fiber counting at each experimental time, per volunteer. Statistical differences were assessed by paired t-test (α = 0.05).
Among the 52 selected participants, 35 completed the 6 months treatment with the investigational product. They were submitted to the medical assessment of clinical signs and discomfort sensations, to the efficacy assessment by phototrichogram and responded to an opinion questionnaire.
Medical assessment of the product safety
None of the 35 participants referred discomfort sensations with the product use and no clinical signs were detected due to the treatment.
Phototrichogram
Figure 1 represents an example of phototrichogram image obtained per experimental time. After analyzing the phototrichogram of the 35 participants we observed that: (1) 27 participants (77% of the panel) presented reduction of the Telogen Effluvium after 3 months of treatment; (2) 25 (71% of the panel) presented an increase in the total number of fibers in the assessed area after 3 months of treatment; (3) 29 (83% of the panel) presented reduction of the Telogen Effluvium and an increase in the total number of fibers after 6 months of treatment. In Figure 2 the percentage of participants with a reduction of telogen hair and increase in the total number of hair fibers per experimental time is shown.
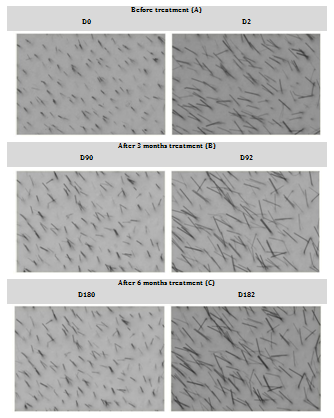
Figure 1 Phototrichogram obtained during treatment with Viviscal®. Legend: Images obtained at the beginning of the study (A) and after 3 (B) and 6 months (C) of treatment.
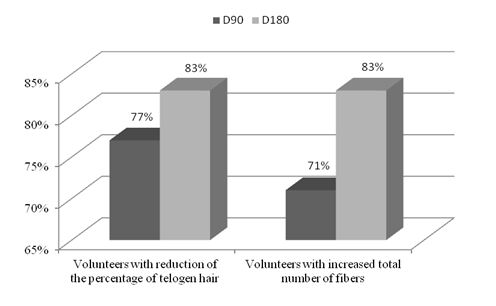
Figure 2 Percentage of participants with an improvement concerning the attributes referred to the Phototrichogram analysis – reduction of the percentage of telogen hair and increase in the total number of fibers.
The telogen fibers density reduced from 11.1 to 8.7 fibers/cm2 and total hair density increased from 110.1 to 118.0 fibers/cm2 after 180 days treatment (Table 2). After 3 months treatment, participants presented 2.5% increase in the total number of fibers and 8.9% reduction in the number of telogen hair fibers. The percentage of telogen hair fibers in relation to the total number of fibers reduced 11.1%. The efficacy was time-dependent since after 180 days of treatment, the total number of fibers increased 7.16%, the reduction in the number of telogen hair fibers was 21.6% and the percentage of telogen fibers reduced 26.9%.
Parameter |
Time (days) |
p-value |
||
D0 |
D90 |
D180 |
||
Total Hair Density |
110.1 ± 21.7 |
112.9 ± 21.0 |
118.0 ± 13.3 |
0.01 |
Telogen Fibers Density |
11.1 ± 3.2 |
10.1 ± 2.7 |
8.7 ± 2.7 |
0.00 |
Table 2 Variation of telogen fibers density (a) and total hair density (b) after 90 and 180 days treatment. Legend: paired t-test statistical analysis was performed for times D0 and D180 (α = 0.05).
Volunteers questioning
The participants were questioned about the impact of hair appearance on their self-esteem and life quality before and after 6 months treatment. They also answered to a product efficacy evaluation questionnaire concerning hair, nails and skin after 3 and 6 months of product use.
The percentage of participants that responded each option to the assessed parameters for the questions concerning life quality is presented in Table 3. Based on the obtained answers we can claim that the volunteers’ life quality improved with the treatment, as the favorability of the answers (% of the answers “not at all” and “irrelevant”) increased on D180 (after 6 months of treatment) when compared to D0 (before treatment).
Questions |
Percentage of Participant’s Answers |
|||||||||
Too much |
A lot |
A little |
Not at all |
Irrelevant |
||||||
D0 |
D180 |
D0 |
D180 |
D0 |
D180 |
D0 |
D180 |
D0 |
D180 |
|
I am ashamed because of my thinning hair |
14 |
3 |
0 |
0 |
23 |
20 |
63 |
77 |
0 |
0 |
Because of my thinning hair I avoid social meetings |
3 |
0 |
0 |
3 |
11 |
3 |
86 |
94 |
0 |
0 |
My condition stops me from eating or drinking on a social event |
0 |
0 |
0 |
0 |
9 |
6 |
91 |
94 |
0 |
0 |
I avoid going out during the day because of my hair loss |
3 |
0 |
3 |
3 |
3 |
3 |
91 |
94 |
0 |
0 |
My condition stops me from participating in sports activities |
0 |
0 |
0 |
0 |
9 |
3 |
91 |
97 |
0 |
0 |
My condition affects my self-esteem |
20 |
0 |
3 |
6 |
29 |
29 |
49 |
66 |
0 |
0 |
I am less confident because of my condition |
3 |
0 |
3 |
3 |
17 |
9 |
77 |
89 |
0 |
0 |
Because of my thinning hair, I fear to be the center of attention |
3 |
0 |
3 |
3 |
6 |
3 |
89 |
94 |
0 |
0 |
Because of my thinning hair, I am less extrovert than I wanted to be |
3 |
0 |
0 |
3 |
3 |
0 |
94 |
97 |
0 |
0 |
Because of my thinning hair I have problems in my intimacy |
6 |
0 |
0 |
0 |
3 |
6 |
91 |
94 |
0 |
0 |
Table 3 Percentage of participant’s answers to the attributes assessed on the life quality questionnaire before and after 6 months treatment.
The criterion to declare a certain answer as relevant in the self-assessment questionnaire was the association of the Top two boxes analysis with the Chi-Square statistic. The Top two boxes analysis considers the frequency of the favorable answers. Therefore, the parameter was considered relevant when at least 66% of the participants responded favorably to the question. Table 4 contains the list of the attributes that presented frequency of favorable responses above 66% per experimental time.
ATTRIBUTE |
D90 |
D180 |
General hair volume |
/ |
94% |
Scalp coverage |
/ |
81% |
Hair thickness |
75% |
92% |
Hair smoothness |
86% |
97% |
Gloss |
86% |
100% |
Nail strength |
78% |
92% |
Nail growth rate |
69% |
91% |
Skin moisture |
/ |
86% |
Face wrinkles |
/ |
74% |
Skin softness |
69% |
92% |
Skin elasticity |
/ |
69% |
Skin smoothness |
75% |
92% |
Healthy skin |
81% |
94% |
Considering all effects observed on my hair, nails and skin, may I claim to be happy with the treatment? |
94% |
100% |
Table 4 Attributes with favorable answers above 66% for the assessment times D90 and D180, referring to the self-assessment questionnaire. Legend: The parameter did not obtain favorable responses above 66% for this evaluation time; D90 = 3 months treatment; D180 = 6 months treatment.
Telogen Effluvium may be caused, among other factors, by nutritional deficiencies, for example, iron, zinc, proteins, vitamins, etc.2,6,14-16 Hair exerts a great impact on the self-esteem and life quality of the population, although it does not possess a vital function.15 For this reason, the development of nutraceutical products with proven efficacy and safety for hair loss treatment is of utmost importance.
The selected phototrichogram technique was adopted because it is not invasive and it does not cause any discomfort for the volunteers. It also allows obtaining images with high resolution, thus enabling a greater reliability in anagen and telogen hair counting.17 On this study, we found that the investigational product Viviscal® was considered safe and effective to reduce Telogen Effluvium. None of the study participants presented topical or systemic adverse reactions to the treatment. We also observed a time-dependent efficacy of the nutraceutical product on the objective assessment by phototrichogram after 3 and 6 months of treatment, as around 70% of the participants reacted to the treatment after 3 months and around 80% after 6 months. After 180 days treatment, the total number of fibers increased 9.3%, the reduction in the number of telogen hair fibers was 19.6% and the percentage of telogen hair fibers reduced 25.6%.
In addition to the objective assessment, we also performed the nutraceutical product’s subjective efficacy evaluation by opinion questioning to the research participants. In this assessment, the product was positively evaluated by the majority of the volunteers regarding the improvement of life quality and parameters related to hair, skin and nails aspect. By the end of the 6 months treatment, 100% of the assessed participants claimed to be satisfied with the treatment’s effects.
Scientific papers demonstrated that Viviscal® supplementation was effective in the treatment of androgenic alopecia in young males,8 was useful for premenopausal women to deal with subclinical hair thinning or hair loss conditions18 and to promote hair growth in adult women with self-perceived thinning hair, according to visual analysis.9,19 However, the phototrichogram technique followed by the image analysis using the software Image Pro Plus® seems more appropriate than visual analysis because it allowed obtaining objective and accurate results.
The Nutraceuticals Viviscal® Maximum Strength Oral Supplement contains acerola fruit blend powder (25% vitamin C), ascorbic acid, AminoMar CTM marine protein complex, a form of silica derived from Equisetum sp. (horsetail), millet dry extract, D-biotin, zinc oxide, ferrous fumarate, and nicotinamide. Nutritional factors such as vitamins and hair loss are highly related. Biotin (vitamin H or B7) is a vitamin that participates as a cofactor in several metabolic pathways, as gluconeogenesis, fatty acid synthesis, and amino acid catabolism. Biotin deficiency is related with neurological manifestations, skin rash, hair loss.15,20 Vitamin C (ascorbic acid) is essential for collagen synthesis and cross-linkage of keratin fibers.6
Zinc is an essential element required by several enzymes and different conditions may affect its absorption and excretion by human body.16 Zinc deficiency is associated with alopecia, hair dryness, brittleness and patients usually responds well to supplementation treatment.16,21 There is no consensus about the relationship between iron deficiency and diseases like alopecia areata, androgenetic alopecia, Telogen Effluvium, and diffuse hair loss,22 but some clinical trials showed that iron supplementation reduced Telogen Effluvium.23
The AminoMar CTM marine protein complex from Viviscal® is composed of a blend of shark powder and mollusk powder, both derived from sustainable marine sources, rich in proteins and glycosaminoglycans of marine origin. Although the exact mechanism of this product is unknown, the reason for the results may be related to greater bioavailability of polysaccharide complexes when compared with similar products. Also, the product may enhance the proliferation of dermal papillae cells, which presents an important role in hair growth cycle.24 As the marine protein complex, the silica is a key component because it is associated with the strengthening of hair. Silica can help to prevent baldness because it stimulates hair growth.25
The research was funded by Lifes2good.
The study procedures were in accordance with ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration.
The author declares no conflict of interest.

©2017 Bloch, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.