Journal of
eISSN: 2373-633X


Research Article Volume 16 Issue 1
1Associate Professor of General Surgery Surgical Academic Unit A, Uruguay
2Resident General Surgery of the CCOU associated with the Surgical Academic Unit A, Uruguay
3Professor Ad Honorem of the Faculty of Medicine of Uruguay, Uruguay
4Prof. of the Surgical Academic Unit A, Uruguay
Correspondence: Ulises Parada, Associate Professor of General Surgery Surgical Academic Unit A, Uruguay
Received: November 26, 2024 | Published: January 17, 2025
Citation: Parada U, Elicegui V, Joaquin P, et al. Gastroesophageal reflux disease and hiatal hernia protocol. J Cancer Prev Curr Res. 2025;16(1):1-10. DOI: 10.15406/jcpcr.2025.16.00569
This chapter comprehensively addresses the fundamental aspects of gastroesophageal reflux disease (GERD) and hiatal hernia (HH), both pathologies often coexisting, significantly affecting the quality of life of patients. The most relevant pathophysiology, diagnosis, therapeutic options, indications and surgical techniques will be analyzed. Advances in medical, endoscopic and surgical treatments are examined, with a particular focus on the types of fundoplications. Likewise, the challenges faced by surgeons when treating patients with GERD and hiatal hernia are discussed, as well as the failure of antireflux surgery, seeking a balance between conservative treatments and invasive interventions to achieve the best long-term results.
Gastroesophageal reflux disease (GERD) is a clinical entity that affects millions of people worldwide, with a significant prevalence, estimated at 2.5 to 33.5% of the general population. GERD is defined as the rise of gastric and/or duodenal contents above the gastroesophageal junction, which causes symptoms and/or lesions that affect the health and quality of life of individuals who suffer from it. It is a multifactorial, chronic, often recurrent condition that requires the intervention of an interdisciplinary team that handles the constant technological advances in diagnosis and treatment. Therapy should be individualized, although lifestyle changes and medication are effective for many patients, but those with severe or complicated forms of the disease should be evaluated for surgical intervention. The advent of minimally invasive surgery in the 1990s has gained acceptance given its obvious advantages. The acquisition of experience and skills has made laparoscopic surgery the approach of choice. Surgical treatment of reflux is based on performing total or partial fundoplication involving the lower esophageal sphincter. The choice between one and the other variant depends on a careful preoperative evaluation. Currently, endoscopic procedures not only play a key role in the diagnosis and evaluation of patients with GERD, but also have an important therapeutic value and offer novel alternatives to medical and surgical treatment. GERD treatment should be viewed as a dynamic and adaptive process. The indication for surgery should take into account the short- and long-term risks and benefits, always prioritizing quality of life and the prevention of severe complications.
There are no prevalence studies in Uruguay to date. The GerdQ questionnaire estimates that this figure ranges from 4.69% to 14.14% when considering only typical symptoms.1
The pathophysiology of GERD is multicausal, where different mechanisms are recognized: dysfunction of the lower esophageal sphincter (LES), anatomical alterations, and alterations in esophageal motility.
The anti-reflux barrier is influenced by two key structures: the LES and the crural diaphragm (Figure 1).

Figure 1 The image above shows the barrier function of the LES, in synergy with the diaphragm and the angle of His. When the LES becomes incompetent (hypotonia, excessive transient relaxations, anatomical alterations) the gastro duodenal contents pass into the esophagus.
LES dysfunction is the most common cause of GERD.1
The diaphragm on the other hand functions as an external sphincter (intra-abdominal location of LES, phrenoesophageal ligament, angle of His).
When these mechanisms fail, the barrier function is lost and the intragastric pressure exceeds the LES pressure, allowing the reflux of gastric contents into the esophagus. The most important factors involved as a possible etiology of this dysfunction are:
They are multiple they are outlined in Table 1. Lifestyle related- obesity (it is considered that approximately 45% of obese people are accompanied by GERD), smoking, and alcohol consumption. Foods that increase acid secretion such as acids, fats, caffeine, chocolate, Comorbidities, it has been observed that patients with Diabetes Mellitus with autonomic neuropathy present a higher percentage of LES dysfunction, predisposing to GERD. It is considered that hormonal and genetic factors may also have an influence. Scleroderma (with reduced contractility or absence of contractions of the smooth muscle of the esophagus due to atrophy of the same together with hypotension of the LES) leads to very significant degrees of GERD. Drugs that relax the LES such as benzodiazepines, calcium antagonists, nitrates among others. Previous surgical interventions that alter, weaken or undo the esophagogastric junction, as can be caused in some circumstances by achalasia surgery, esophagogastric anastomoses with removal of the EG junction, and operations that act on the esophageal hiatus, can also promote the appearance of reflux.
|
Lifestyle |
Comorbidity |
Others |
|
Smoking |
Obesity |
Pregnancy |
|
Alcohol intake |
Diabetes |
Drugs: benzodiazepines, calcium antagonists, nitrates, among others. |
|
Diet: fat, caffeine, chocolate |
Connective tissue diseases: systemic sclerosis, scleroderma, Sjögren's syndrome |
|
Table 1 Risk factors for gastroesophageal reflux disease
Reflux esophagitis is the lesion produced by the contact of the refluxed content in the esophageal mucosa and causes epithelial destruction followed by scarring. The classic histological criteria for diagnosing esophagitis are of little use in establishing the significance of esophagitis. Although the modified Savary-Miller classification has been classically used, the most widely accepted classification of esophagitis is currently the Los Angeles classification. Sometimes, the demonstration of the damage caused by GERD is only histological and consists of an increase in the thickness of the basal cell layer of the epithelium, the called “occult esophagitis (Table 2).2 On the other hand, Savary-Miller grade IV includes ulcers and stenosis which are very rare today. The ulcer can acquire own evolutionary capacity, and may bleed, penetrate into neighbouring structures or perforate. Due to its severe fibrosis, the stenosis may be accompanied by oesophageal shortening.
|
Grade A |
1 or more erosions less than or equal to 5 mm in length that do not extend between the upper ends of the folds of the esophageal mucosa. |
|
Grade B |
1 or more erosions more than 5 mm in length but not continue between the upper ends of the folds of the esophageal mucosa |
|
Grade C |
1 or more mucosal erosions that continue between the ends of the folds of the esophageal mucosa that They involve less than 75% of the esophageal circumference. |
|
Grade D |
Erosions affecting more than 75% of the circumference esophageal. |
|
Grade E |
Ulcers, strictures, Barrett's esophagus. |
Table 2 Los Angeles classification
At the level of the esophageal body, esophageal intraluminal manometry (EIM) has shown that, with
GERD is often associated with peristaltic dysfunction of the esophageal body, Two different motor patterns have been described. Sometimes it is a failure of primary peristalsis (absence of motor response to swallowing) and/or a decrease in the amplitude of the contractions, which are often simultaneous. At the level of the body of the esophagus, a smaller group of patients present another type of motor disorder, which may have clinical prominence and is characterized by the presence of high amplitude contractions that may be simultaneous and repetitive in a high percentage of swallowings, giving rise to the so-called “diffuse esophageal spasm secondary to GERD”. It is unknown whether these motor disorders appear as a consequence of GERD or, on the contrary, they are part of the etiology of GERD. In this regard, some authors have reported the improvement of peristaltic dysfunction of the first type after healing of esophagitis, which would support its secondary nature. At the EES level, It has been seen that in a high number of patients with GERD there is a hypertensive upper esophageal sphincter (UES), a hypotensive UES and sometimes swallowing abnormalities (such as pharyngo-sphincteric asynergia, which is related to Zenker's diverticula).
They are very common in pediatric patients and are due to the passage of refluxed material into the airway. They include acute asphyxia crises due to massive passage, recurrent laryngitis, recurrent pneumonia, bronchitis, pulmonary fibrosis and asthma, chronic intractable asthma. In relation to the latter, several studies have shown that most adult asthmatics have pathological GERD and that antireflux treatment (medical or surgical) may be beneficial in patients who also have GERD symptoms.
It is an incomplete esophageal membrane located at the level of the junction of the esophageal and gastric mucosa, such that its upper surface is covered by squamous epithelium and the lower by gastric epithelium. Its congenital nature has been discussed and it is accepted that it is a consequence of GERD. It may not cause symptoms, although sometimes, with very solid food boluses, it can cause dysphagia and even food impaction. Radiologically, the image is typical and endoscopy is usually clearly visible, and in almost no case does it offer resistance to the passage of the endoscope. If it causes dysphagia, the treatment is endoscopic dilation.
Diagnostic evaluation of GERD is essential to direct the therapeutic strategy since the success of the assigned treatment is based on correct patient selection.2 This includes a combination of clinical, upper gastrointestinal endoscopy (UGE), as well as imaging and functional studies (manometry and pHmetry).
Many of the symptoms may be insensitive and non-specific, so a thorough and detailed anamnesis is essential. It allows identifying characteristic symptoms, risk factors, ruling out complications, and directing the request for diagnostic tests, thus selecting the patient appropriately, reducing hospital costs and unnecessary studies. As for symptoms, there are typical and atypical symptoms.
Heartburn and regurgitation (mostly postprandial or in the supine position and improved by the administration of antacids) are present in 70-90% of patients. The fundamental symptom is heartburn, which consists of a burning sensation that ascends and descends from the epigastrium to the cervical region behind the sternum. Another very frequent symptom is regurgitation of gastric contents into the mouth, which is usually acidic. These regurgitations can cause irritating coughing attacks and even bronchopulmonary infections, due to the passage of the regurgitated material into the airway. Sometimes, the air content of the stomach ascends into the esophagus through an incompetent LES and comes out as repetitive belching.
Pain with swallowing odynophagia usually reveals the presence of mucosal ulcerations, so it is often associated with severe esophagitis. It requires ruling out infectious esophagitis and ulcerated esophageal cancer. The appearance of dysphagia of a logical, continuous and progressive nature should lead to the suspicion of esophageal stenosis due to reflux. If the dysphagia is intermittent in nature, it could be due to esophageal motor dysfunction. Chest pain it is the least specific symptom of GERD and seems to be more associated with motor disorders. It can occur in crises that resemble, due to their intensity and location, coronary pain, from which it must be differentiated. Chronic anemia syndrome is sometimes present. It is usually the result of large, mixed hiatal hernias (with a paraesophageal component). Other atypical clinical data include a sensation of constriction at the cervical level and those related to pulmonary complications (recurrent pneumonia, asthma, pulmonary fibrosis, etc.) and laryngeal complications (chronic laryngitis, dysphonia).
In patients with GERD, it is important to differentiate between three diagnostic aspects: sliding hiatal hernia (SHH) as an anatomical alteration, gastroesophageal reflux (GER) as a pathophysiological disorder, and finally, the esophageal and extraesophageal repercussions of GERD. Knowledge of all these data is essential to decide on the most appropriate treatment. The anamnesis is essential to diagnose GERD and its consequences, so its primary category must be clear. However, a series of complementary examinations are currently available that can effectively contribute to the solution of each of these three diagnostic problems (Table 3).
It presents precise indications: typical symptoms of GERD that do not respond to well-established medical treatment, after anti-reflux surgical procedures, clinical complications, or alarm elements. This is a widely requested study to assess the presence of esophagitis, the severity of GERD, and to take biopsy samples. It has a specificity of 90%, but its sensitivity is low; 50% of the affected patients may not express pathological elements. There is no anatomical-clinical correlation between the intensity of the symptoms and the characteristics of the endoscopic lesions. PPIs should be discontinued 2 weeks prior to performance. Biopsies will be taken if Barrett's esophagus (BE) is suspected (present in 10% of patients with GERD undergoing endoscopy), eosinophilic esophagitis, suspected neoplasia, or infections. In the presence of symptoms that do not improve, a control VGC will be performed, after the use of at least 8 weeks of double-dose PPI. It will also be performed in the case of severe esophagitis, in order to rule out its progression to BE. Chromoendoscopy describes structures observed with white light. It is relevant in non-erosive GERD, directly observing the increased submucosal vasculature above the squamous cell junction. It allows the detection of minor changes and dysplastic or neoplastic lesions, but it is not widely used in our setting.2
A simple chest X-ray, from the front and from the side, can provide images (air spots, opacities, etc.) that show the intrathoracic stomach. This will only be seen in large or irreducible hernias. Images corresponding to pulmonary complications due to aspiration can also be seen, especially in children. Esophagogastric bypass (EGB) with barium swallow is the most useful examination for diagnosing hiatal hernia as an anatomical abnormality. The EBG may also be useful for diagnosing the existence of GERD, by demonstrating that the barium swallow ascends from the stomach into the esophagus, although it is not the most appropriate test. Regarding the consequences of GERD in the esophagus, it can reveal images of peptic stenosis, motor disorders of the esophageal body and the Schatzki ring.
It is a useful tool for assessing LES function and esophageal motility. Normal resting pressure typically lies in the range of 10 to 45 mmHg. This pressure varies slightly between individuals and can be affected by factors such as body position and food intake. The following conditions are considered pathological:
MIE is the only test that allows the diagnosis of motor abnormalities that may occur in reflux. However, there are other factors involved in the appearance of GERD, so there is no limiting value for LES pressure that allows patients with and without GERD to be separated. There is a finding at the LES level that suggests the presence of a hiatal hernia: the recording of a high pressure zone (HPZ) that is related to the existence of a double pressure peak (manometric data typical of a hiatal hernia). Furthermore, it is the only method to demonstrate the existence of a body, esophageal without peristaltic activity due to scleroderma, achalasia, etc., circumstances of great use when focusing on the treatment of these patients. In patients with chest pain and/or dysphagia, especially if endoscopy is normal, it is essential to do so. Finally, to perform the pH test, it is necessary to first perform manometry to locate the LES. On the other hand, it adds less to the knowledge of GERD.
It is especially useful in patients with atypical symptoms of GERD or in those in whom endoscopy reveals no abnormalities, and in patients who do not respond to medical treatment. It is considered the “gold standard” for the diagnosis of patients with pathological acid GERD, since it allows objectification of esophageal acid exposure. It has limitations such as sensitivity close to 60%. Technical considerations: Antihistamines should be discontinued 2 days prior, PPI 1 week prior. The distal sensor should be placed 5 cm proximal to the LES (must be located by manometry). The following must be measured:
Recently, the use of multichannel intraluminal impedance combined with PH (PH-IIM) allows detection of reflux characteristics at all pH levels (acidic and non-acidic), a potentially useful feature in the assessment of persistent symptoms despite acid suppression.
The aim of medical treatment is to improve symptoms and thus the quality of life of patients. In most patients, it prevents the progression of the disease as well as its complications.1,2 To this end, emphasis will be placed on lifestyle changes, discouraging the aforementioned risk factors, in addition to hygienic-dietary changes such as:
Drug treatment is mainly based on proton pump inhibitors (PPIs), which have demonstrated their superiority over H2 receptor inhibitors (famotidine, ranitidine). The most commonly used PPIs are: omeprazole, pantoprazole, esomeprazole, lansoprazole, etc. There is currently no consensus on which to choose and their doses. However, the Uruguayan Society of Gastroenterology recommends them, empirically, at standard doses (20 mg per day) for 4 weeks if the patient presents with typical symptoms, and 12 weeks in patients who associate extraesophageal symptoms. In patients with confirmed GERD with esophagitis, the recommended dose is 4 to 8 weeks, depending on the severity of the esophagitis. In severe esophagitis, the recommended dose is doubled (40 mg per day). The patient should be instructed and its administration should be indicated in the mornings 30-60 minutes before breakfast and/or dinner. There is controversy over the use of PPI treatment and possible side effects, but in general the risk of discontinuing them is greater than leaving esophagitis untreated when they are indicated. Oral antacids are not effective against healing esophagitis, but they can quickly relieve symptoms. They are only used for short periods. Sucralfate may be helpful in esophagitis. Prokinetics (increasing lower esophageal sphincter pressure, improving esophageal clearance and gastric emptying) may be used in patients who also have bile reflux or delayed gastric emptying. However, a significant percentage of cases present undesirable side effects.
Correct selection of candidates for antireflux surgery is essential for the procedure to be successful. As already mentioned, this decision will be individualized and based on a thorough medical history and diagnostic methods. Several predictors of good results of antireflux surgery have been identified. Patients with typical symptoms, good response to PPIs, BMI less than 35, and young age have better results. On the other hand, those who show atypical symptoms and poor response to PPIs have less promising results. It is essential to communicate this information to the patient before proceeding with surgery, in order not to generate false expectations of treatment, considering the risks and benefits of the surgery.
Surgical techniques for treating reflux have evolved significantly over the last few decades. It should be noted that initially, the main focus was on treating hiatal hernia and complications of reflux, such as esophageal shortening and stenosis. The lack of effective medical treatment led to a considerable percentage of cases with these conditions being operated on. The advent of PPIs led to a significant decrease in these interventions. Subsequently, reliable methods for studying reflux and fundoplication techniques clearly changed the results. Finally, the introduction of laparoscopic techniques with the demonstration of excellent results with minimal complications led to the current situation. All this should not hide the fact that surgical indication based on the tests and preoperative diagnostic study already discussed is essential. Currently, fundoplication techniques and their variants are mainly used to treat reflux surgically. The most important ones are Figure 2.
Collis: technique is a gastroplasty that lengthens the esophagus at the expense of the lesser curvature of the stomach. It is useful in cases where it is not possible to return the lower esophagus to the abdomen without tension, usually due to severe esophagitis. It can be performed in combination with a fundoplication (Collis-Nissen). Many patients present concomitantly with hiatal hernia. Treatment of this is mandatory when both entities are present, by reintroducing the sac and its contents (Figure 2 & 3). Most of these operations are now performed using a laparoscopic approach. Time has shown the advantages of this technique in terms of a lower incidence of complications, good short- and long-term results, shorter hospital stays, less need for analgesia, and the well-known aesthetic advantages. If it is performed by a team with experience in this pathology, the advantages are unquestionable. Performing this operation using a robot is also advocated, although the advantages over laparoscopy are minimal and the cost is higher. 3–5
Understanding the impact that surgical treatment has on the quality of life of patients is essential. There are many questionnaires that attempt to objectify the impact it causes, knowing that it has a subjective part. In addition, these questionnaires serve as an “audit” of response to surgical treatment. These include GERD-HRQL (Gastroesophageal Reflux Disease Health Related Quality of Life) and the RDQ (Reflux Disease Questionnaire). The influence of psychological disorders was studied years ago, especially using the GHQ-28 (General Health Questionnaire-28) questionnaire in patients who underwent laparoscopic GERD surgery. In addition to systematic functional studies, these patients were also given questionnaires (SF-36 Gastrointestinal Quality of Life Index and the GHQ-28) before and after surgery. Although all patients experienced an improvement in their quality of life, no differences were found after surgery in functional results and in degree of satisfaction between cases with a normal or pathological GHQ-28 questionnaire. However, patients with a pathological result in the GHQ-28 had worse results in all domains of the SF36-Gastrointestinal Quality of Life compared to patients with a normal preoperative GHQ-28 questionnaire. This decrease in the degree of quality of life did not have an impact on the degree of satisfaction with the surgery performed. Thus, the GHQ-28 does not seem to be a good predictor of the degree of postoperative satisfaction.6–8
The following endoscopic procedures attempt to offer alternatives to the medical and surgical treatment described above in patients without a large hiatal hernia. They require careful case selection, and improvements in these devices are gradually appearing, although long-term follow-ups of treated cases are necessary. They are promising and may be indicated for patients with mild to moderate GERD who do not wish to undergo surgery or who have a high anesthetic-surgical risk. They are not indicated for severe GERD. They require adequate equipment and a sufficiently trained medical team, as well as the relevant learning curve.
Performs an endoscopic fundoplication orally to create a valve mechanism with a high pressure zone of approximately 3 cm in the distal esophagus below the diaphragm, at 200 to 300º, using an endoscopic suturing instrument. Improves reflux symptoms and acid exposure time, reducing PPI consumption.
Designed as an instrument that combines vision, ultrasound and a surgical stapler and that allows an anterior fundoplication via an endoscopic transoral route.
Endoscopically guided, it applies radiofrequency to the muscle fibers of the LES and the gastric cardia and stimulates collagen production, strengthening the anti-reflux barrier. A recent meta-analysis somewhat questions its results.
In the treatment of GERD, MSA is a laparoscopic surgical procedure in which previously well-documented reflux is treated by augmenting the esophagogastric junction barrier by placing a magnetic ring of titanium pieces. It seems to be an attractive treatment for patients with well-studied reflux who prefer to avoid very long-term medical treatment, or in whom symptoms are not adequately treated with lifestyle measures and medical therapy. Cases with a lot of symptoms of regurgitation, and without dysphagia or motor disorders, seem to be better candidates. Suitable candidates with significant hiatal hernia can be treated with simultaneous hernia repair. Comparative studies with laparoscopic Nissen report a lower incidence of dysphagia, as well as difficulty vomiting and belching. However, there are also cases of dysphagia that appears to be related to the diameter of the ring used and that may even require surgery reoperate.
Causes that may cause GERD9 treatment interventions to fail include:
Sometimes it is not easy to identify these causes exactly despite thorough studies, and in a practical way and with a view to the therapeutic approach they can be grouped into these four:
Regarding the indications for surgical treatment of failed GERD10 surgery, it is important to take into account several considerations: Most Nissen with poor outcomes can be treated quite well conservatively.
In the few cases that require reoperation, the result is somewhat inferior to that obtained with the first operation (50-89%), with a lower incidence of asymptomatic patients and somewhat higher morbidity and mortality. The 2nd and 3rd reoperation decreases the success rate (20% with each new operation). In addition, the most problematic cases for indicating or not indicating surgery are:
For all the reasons stated above, experience shows that these reinterventions should ideally be performed in specialized centers,10 and that the surgeon is a fundamental prognostic factor (he must be well acquainted with the pathophysiology, have experience in these reinterventions by laparoscopy, laparotomy and thoracotomy, as well as in esophagectomies).
The interactions of certain comorbidities such as diabetes with the gastrointestinal system and LES function mean that certain considerations must not be overlooked in the management of these patients.
In patients with other comorbidities, such as hypertension, obesity, cardiovascular disease, or respiratory diseases, GERD management should be tailored to the patient's specific conditions.
Barrett's esophagus (BE) is defined as the replacement of normal esophageal stratified squamous epithelium by simple columnar epithelium. Endoscopically: It is identified as a salmon-colored lesion with a circumferential or islet-like tab pattern in the tubular esophagus > 1 cm. Histologically: intestinal-type metaplasia. It is an acquired premalignant disease in response to gastroduodenal reflux that predisposes to adenocarcinoma. Only a small group of patients develop EB and the minority of these will develop adenocarcinoma. According to its length, EB is classified as short segment: less than 3 cm and long segment: greater than 3 cm. The importance of this classification lies in its progression and response to treatment. There is also ultra-short EB, in the form of islands with intestinal epithelium without endoscopic evidence of columnar metaplasia, of questionable usefulness, unrelated to GERD, and of unclear significance.
Approximately 1% of patients who attend endoscopy services have BE, this figure increases to 5% if we consider consultations for symptoms of gastroesophageal reflux.
The following factors are considered:
Risk factors for generating dysplasia
Although there are risk factors, there are also protective factors such as proton pump inhibitors, which reduce the risk of developing dysplasia and esophageal adenocarcinoma by 71%. Medications such as cyclooxygenase inhibitors, such as aspirin and statins, also reduce the risk of displasia (Table 3).
|
Risk of progression to adenocarcinoma Esophageal or high-grade dysplasia |
Incidence % |
|
Without Barrett's disease |
0.33 |
|
Barrett's disease short without dysplasia |
0.19 |
|
Barrett's disease with low-grade dysplasia |
0.5/1.7 |
|
Barrett's disease with high-grade dysplasia |
7 |
Table 3 Risk of progression to esophageal adenocarcinoma, dysplasia stands out as the only determining marker in the risk of progression to esophageal adenocarcinoma
Video gastroscopy, Typically, an alteration is observed in the esophageal mucosa with a salmon-reddish color with a distribution in tabs, circumferential or in islets included in healthy mucosa. The video endoscopy report should include:
It is very important to systematically take biopsies in EBs >3cm in the four quadrants every 2cm, and in those <3cm every 1cm, noting each sample separately as a reference. Also taking biopsies of the squamous epithelium above the Z line.
Due to the frequent association with GERD, other tests are usually performed: esophageal-gastro-duodenal radiographic transit, esophageal manometry, 24-hour pH monitoring. Occasionally, and for a more complete study, monitoring of bilirubin in the esophagus may be indicated (Bilitec 2000). The risk of malignant transformation of EB is considered to be present, but low, approximately 0.2% per patient per year, but with great variability between series, which would not justify an indiscriminate early detection program, but rather endoscopic surveillance of cases with a higher chance of malignant transformation. The most important is the presence of dysplasia in biopsies, even admitting the difficulty of making a precise determination in low-grade biopsies. Other factors to consider are the risk factors mentioned above, together with severe GERD (acid and biliary), failure of medical treatment, and familial EB or a history of esophageal adenocarcinoma. Molecular and genetic alterations are studied, although these techniques are complex to perform, poorly standardized, and not very useful at the moment in daily clinical practice. metaplasia-dysplasia-adenocarcinoma is accepted, as in the colon. Although histologically, EB is associated with esophagitis in all cases, inflammatory changes are only visualized in endoscopy in 30 to 80% of cases and may affect the esophageal mucosa and the metaplastic mucosa. In a considerable percentage of cases, strictures Esophageal complications occur at the transition line from the EB to the squamous epithelium (20 to 50% of cases). Occasionally, Barrett's ulcer is seen, usually in the metaplastic segment. All of these complications are favorably influenced by intensive medical treatment with PPIs (Figure 4).
The most common is that the patient does not have symptoms or symptoms of reflux. In this situation, medical treatment or anti-reflux surgery can be considered. With medical treatment, symptoms are usually well controlled, and it can be considered for life, but the important thing is to avoid or detect the appearance of dysplasia or adenocarcinoma, and it is very controversial whether this is better achieved with anti-reflux treatment or surgery. The first has the advantage of avoiding surgery and usually the symptoms, the second is more aggressive, but if performed properly it manages to control not only acid reflux but also alkaline reflux. There are studies that seem to indicate that correctly performed reflux surgery seems to protect better against malignancy than conservative treatment. As for the surgical technique to be performed, it is universally accepted that Nissen fundoplication is effective for common cases provided that an adequate segment of abdominal esophagus is achieved without tension. If there is stenosis, they usually respond well to prior treatment with PPI. If a tension-free abdominal esophagus cannot be achieved, the Collis-Nissen technique is recommended. If the patient has already undergone previous surgery, dissection of the esophagogastric region is highly risky and reflux persists, the duodenal diversion technique may be considered. If there is significant esophageal stenosis or high-grade dysplasia persists after intensive PPI treatment, subtotal esophagectomy will be considered, usually with a high anastomosis above the azygos arch and without the need for lymphadenectomy. Since neither medical nor surgical treatment causes the metaplastic mucosa to disappear, other treatments have been attempted (electrocoagulation, laser, etc.) but all have drawbacks. However, the treatment that seems effective in making the metaplasia disappear is radiofrequency (HALO) without the complications and problems of the others and which can be combined with anti-reflux surgery. In cases with high-grade dysplasia, after confirming it with intensive medical treatment and new biopsies with the participation of another pathologist, mucosal ablation, radiofrequency and monitoring, or directly subtotal esophagectomy can be considered (among other things due to the percentage of these cases with adenocarcinoma in the removed specimen).11
Hiatal hernia is defined as any passage of abdominal contents into the thorax, through the esophageal hiatus, accompanied by a peritoneal sac.
There are 4 types among them:
Most HH are asymptomatic, which makes it difficult to determine their exact incidence, but in the West it is around 15 to 20%. Clinical symptoms are variable and not very specific: dysphagia, chest pain, postprandial fullness. Nausea, vomiting, heartburn and regurgitation. In a significant number of cases it is associated with GERD. Other associated symptoms are: The presence of hypochromic microcytic anemia may be present due to in apparent bleeding from the gastric mucosa. Symptoms of a mechanical component, digestive intolerance, or sudden retrosternal pain may be the result of a complication of hiatal hernia. They are more common in women between 50 and 70 years of age. Their pathogenesis is not entirely clear, but factors such as obesity, advanced age and increased intra-abdominal pressure play a role.12
Although both conditions can coexist as has been proposed so far, one can perfectly occur without the other. How does HH influence the appearance of GERD? HH basically produces an anatomical decoupling of the barrier mechanism. When the LES is displaced towards the thorax, the positive intra-abdominal resting pressure is lost, the hiatus does not fulfill its function as an external sphincter since it is dilated and does not sit on the LES (displaced towards the thorax with negative pressure). Additionally, the angle of His is lost, which facilitates the passage of gastric contents into the esophagus. From the previous pathophysiological analysis and the types of hernia described, it can be deduced that HH types I and III are more associated with GERD and, on the contrary, type II, which present the cardia in its anatomical position, are associated with mechanical symptoms such as pain and obstruction (Figure 7).
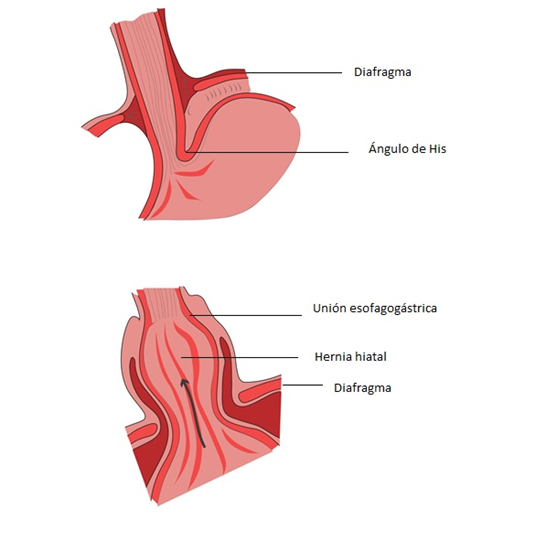
Figure 7 Mechanism of failure of the antireflux barrier in hiatal hernia, the upper image shows the intra-abdominal lower esophageal sphincter below the diaphragm subjected to positive pressure, with the His angle preserved, the lower image shows a hiatal hernia in which the anatomical barrier mechanism is dismantled: the sphincter passes into the thorax, loses resting pressure and the diaphragmatic hiatus is dilated, losing its function as an external sphincter.
Diagnosis is confirmed by upper gastrointestinal endoscopy and imaging methods already described. It is worth highlighting the performance of a contrast study, to visualize the type of hernia and its topography. Manometry and pHmetry have already been described. They will be necessary to confirm the association with GERD, and fundamentally to topography the LES. A computed tomography of the chest and abdomen allows the size of the hernia and its contents to be assessed, and in emergencies it allows the visualization of elements of strangulation: air-fluid level, hypoperfusion of the stomach walls, even perforation, pneumoperitoneum.
In type II, III and IV hernias, surgery is indicated, especially if they are symptomatic. In type I HH, the indication is given by the presence of GERD.
The opportunity for surgery should be carefully assessed. We know that the morbidity and mortality associated with elective surgery is low in experienced teams, whereas emergency surgery carries a high morbidity and mortality rate (up to 37% in some series). Therefore, a correct preoperative assessment should be carried out, in conjunction with the cardiologist and anesthesiologist, in order to reduce the morbidity and mortality associated with elective surgery. The gold standard approach is laparoscopy. This type of repair allows good access and visualization of the hiatus, allowing for a feasible and safe repair. However, in large hernias, maneuverability of the contents may be difficult. Hernia recurrence varies between 5 and 10%. The objective of the technique will be the reduction of the hernial sac and its contents, associated with an antireflux procedure. The removal of the hernial sac, whenever possible, prevents the risk of intrathoracic collections and reduces recurrences. In some cases, with large hernias, the sac can be partially removed to avoid iatrogenic events such as pleural or pulmonary lesions, hemorrhages, etc. But always with the aim of achieving a tension-free abdominal esophagus and adequate dissection of both branches of the diaphragmatic pillar. Then, both branches of the diaphragmatic pillar are closed around the esophagus, and a fundoplication is generally performed using the Nissen type. Nowadays, in large hernias with a very wide hiatus and in elderly patients, it is preferred to add to the above the placement of a reinforcing mesh, with biological mesh being preferable. Although recurrences are described, it seems that they are less frequent and, in a considerable part, asymptomatic (Table 4).
|
Laparoscopic approach |
It is the method of choice, it has the advantages of minimally invasive approaches, it is feasible and safe. |
|
Reduction of the contents and removal of the hernial sac |
Prevents collections and reduces recurrences. |
|
Achieve 2-3 cm abdominal esophagus |
Reduces risk of chest rise |
|
Closure of both branches of the pillar |
In the retrogastric sector (fibrous), closure with non-absorbable “X” stitches to distribute tension and prevent tearing. Key point with high technical demand. |
|
Fundoplication |
Anti-reflux barrier, serves as an anchor at the abdominal level |
|
Primary closure or prosthetic closure |
Mesh closure is controversial, with different technical options described and reserved for large defects between the pillars. Current evidence refers to them as safe and that they may reduce recurrence. |
Table 4 Technical steps in hiatal hernia repair
The diagnostic and therapeutic complexity of GERD and HH, as well as their possible complications, make it essential to create specialized interdisciplinary units that have the necessary technology and trained personnel to provide safe and quality care. Complementary treatment, which is by no means exclusive of these conditions, is based on a combination of lifestyle modifications, medications that reduce gastric acidity and, in some cases, endoscopic and/or surgical intervention. Early identification and adequate treatment, as well as strict monitoring, are key to improving patient well-being and preventing long-term complications. The challenge lies in providing efficient, accessible and effective care, tailored to the individuality of each patient. This can only be provided by professionals who take care of every little detail, with a true work where “the whole is greater than the sum of its parts.
None.
The authors declare that there are no conflicts of interest.

©2025 Parada, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.